Turmeric stains – a fact that is made clear to me every time I make curry and a drop ends up on my light coloured counter tops (what were the previous owners thinking when they installed baby blue counter tops?). Turmeric seems to stain more than any other spice I use, why?
First, a bit about turmeric. Native to South Asia, turmeric plants thrive in the moist, hot conditions found there. It's a member of a tasty family including: ginger, galangal and cardamom. The name 'turmeric' may originate from the Latin terra merita, which means merit of the earth. The turmeric powder commonly used as a spice is derived from the rhizome, a horizontal stem that typically grows shallow beneath the soil. From this stem, roots and shoots are sent out. These rhizomes are harvested about nine months after planting, then boiled, peeled and dried in the sun. Once dry, it is ground into a power.
About turmeric, Marco Polo said “There is also a vegetable which has all the properties of true saffron, as well as the smell and the colour, and yet it is not really saffron.” In medieval Europe, this spice was called 'Indian saffron' and was commonly used as an alternative to the expensive saffron. According to 'The Flavor Bible' turmeric has a bittersweet pungent flavour. Today it's commonly used to make mustard yellow, and as a component in curries that adds both flavour and colour. At times, turmeric even been used to colour cheeses, margarine and chicken broth. I wouldn't mind at all if turmeric was used as a colourant in my iron pills because it can do some good. In fact, a long list of potential medicinal uses have been attributed to turmeric, one use that has been proven is that it reduces inflammation.
Turmeric's use as a dye probably dates back as long as it's been used. I can't imagine not to noticing that turmeric stains cooking implements once it's added to a dish, however, the first record of using turmeric as a dye comes from an ancient Assyrian herbal recipe dating back to 600 BC (a fact from The Cook's Encyclopedia of Spices). The yellow colour is caused by curcumin, a chemical component of turmeric. About 5% of the dry powder is curcumin. The colouring components of other spices like paprika are less than 1%, so, to answer why turmeric stains more, there is just more colouring potential in the turmeric. On the plus side, turmeric fades in sunlight – so if a drop of curry ends up on a favorite white shirt put it in the sun for the colour to fade.
Monday, December 27, 2010
Friday, December 17, 2010
Can we come up with a better way?
I've been thinking a lot lately about ways I can reduce my ecological footprint and I have some ideas.
I've been reading 'Water, A Turbulent History' by Stephen Halliday, a book I randomly found in the library. I was looking for books on how to improve my house's energy efficiency in the engineering section and I found a history book. I was intrigued by the title so I signed it out. How he describes water pollution by sewage got me thinking.
In 1357, King Edward III decreed 'no man shall take any manner of rubbish, earth, gravel or dung out of his stables or elsewhere to throw and put the same into rivers Thames or Fleet'. Due to this attitude, up until the early 1800's, the Thames and its tributaries remained reasonably clear of pollution.
In London during these times, human waste was removed by dedicated workers called 'nightsoilmen'. These men carted away the waste at night and sold it to farmers as manure. Apparently, they earned a decent pay for their work. Then things changed for the nightsoilmen. Competition was introduced when bird guano was imported from South America, starting about 1840. The guano was easier to work with for the farmers and had less of a smell. About this time, having a water closet in your home became a status symbol.
Water closets were invented in the 16th century by Sir John Harington, who made two. One of which was given to Queen Elizabeth I. She didn't like it because the loudness of the flushing announced to everyone when she used it. Without a royal stamp of approval, the water closet became a neglected idea until early in the nineteenth century.
When a water closet is flushed there is only a little 'waste' for 10-20 times the volume of water. The nightsoilmen found this wet waste hard to collect and transport and the farmers no longer wanted it. Cesspools now overflowed into the waterway, polluting them. Cholera epidemics followed and ultimately sewer systems were built.
So now what was once composted (and likely still is in many parts of the world) is now diluted with water and washed away through dedicated pipes buried beneath the ground by gas guzzling equipment. Sewage ultimately ends up at a dedicated treatment plant of some sort that no one wants to be a neighbour to. At any point a leak could pollute our water ways and in many places this has happened. There must be a solution for urban dwellers that doesn't require fancy infrastructure or lots of a critical resource such as water. I realize human waste can carry disease, but is watering it down and flushing it away the best way to deal with it?
I've been reading 'Water, A Turbulent History' by Stephen Halliday, a book I randomly found in the library. I was looking for books on how to improve my house's energy efficiency in the engineering section and I found a history book. I was intrigued by the title so I signed it out. How he describes water pollution by sewage got me thinking.
In 1357, King Edward III decreed 'no man shall take any manner of rubbish, earth, gravel or dung out of his stables or elsewhere to throw and put the same into rivers Thames or Fleet'. Due to this attitude, up until the early 1800's, the Thames and its tributaries remained reasonably clear of pollution.
In London during these times, human waste was removed by dedicated workers called 'nightsoilmen'. These men carted away the waste at night and sold it to farmers as manure. Apparently, they earned a decent pay for their work. Then things changed for the nightsoilmen. Competition was introduced when bird guano was imported from South America, starting about 1840. The guano was easier to work with for the farmers and had less of a smell. About this time, having a water closet in your home became a status symbol.
Water closets were invented in the 16th century by Sir John Harington, who made two. One of which was given to Queen Elizabeth I. She didn't like it because the loudness of the flushing announced to everyone when she used it. Without a royal stamp of approval, the water closet became a neglected idea until early in the nineteenth century.
When a water closet is flushed there is only a little 'waste' for 10-20 times the volume of water. The nightsoilmen found this wet waste hard to collect and transport and the farmers no longer wanted it. Cesspools now overflowed into the waterway, polluting them. Cholera epidemics followed and ultimately sewer systems were built.
So now what was once composted (and likely still is in many parts of the world) is now diluted with water and washed away through dedicated pipes buried beneath the ground by gas guzzling equipment. Sewage ultimately ends up at a dedicated treatment plant of some sort that no one wants to be a neighbour to. At any point a leak could pollute our water ways and in many places this has happened. There must be a solution for urban dwellers that doesn't require fancy infrastructure or lots of a critical resource such as water. I realize human waste can carry disease, but is watering it down and flushing it away the best way to deal with it?
Tuesday, December 14, 2010
Orange poo and red hats

It's officially a winter day where I live – it's raining. My backyard has turned into a monster mud pit. The sky is gray and everything else ranges from muddy green to brown. But, there is some colour – you just need to look closely. Close to home, the holly bush over my black fence is sporting a few red berries. In the forest there is other colourful natural stuff to be found, even this time of year. Looking through my photos of last winter I found these two colourful gems.
One of my favorites is what I always called 'orange poo'. I remember finding it the woods where I grew up and thinking it was poo of some magical creature. Anyway, by consulting my 'Common Mushrooms of the Northwest' book, I've found it called 'orange jelly' or Dacrymyces palmatus. It's a fungi and apparently edible but not too tasty – I'm not going to confirm this as I have a general policy of not eating wild mushrooms and this particular on is associated with poo in my mind.
 If you look closely at a rotting log, you might find a tiny hit of red from the British Soldier lichen or Cladonia floerkeanna (this one I found in my 'Mosses, Lichens and Ferns of the Northwest North America' book). To me this lichen looks like a match.
If you look closely at a rotting log, you might find a tiny hit of red from the British Soldier lichen or Cladonia floerkeanna (this one I found in my 'Mosses, Lichens and Ferns of the Northwest North America' book). To me this lichen looks like a match. In general, a lichen is a fungi that is living symbiotically with some sort of alga. Lichens can survive in all sorts of extreme places – some even survived being exposed to outer space for over two weeks (yes, an experiment was conducted on this). Reading the introduction section of my the lichen section of my book it says lichens are used to dye cloth, ferment beer, and in perfumes, lotions and toothpaste – some can even be eaten.
Even on a winter walk, if you observe carefully, there are all sorts of interesting colourful things to see.
I took both photos while on walks last winter
Thursday, December 9, 2010
Red moon, blue moon
Based on my recent discussion of blues (colours, not moods), I though I should describe what a blue moon is. 'Once in a blue moon' is a common phrase for an uncommon event. However, the moon is never blue (unless there are smoke or dust particles in the atmosphere, then the moon can appear bluish).
I've seen the moon turn a blood red – a frightening event if I didn't know why it changed colour. On that night it was a lunar eclipse. The earth had moved between the moon and sun, casting the moon into its shadow. Normally, the moon reflects sunlight directly from the sun making it a bright feature in the night sky. When the earth is in the way, the only sunlight to reach the moon is refracted around the earth and as a result the moon takes on a blood red colour (at least it did on the night I watched).
A blue moon is a completly different event, more of a bookkeeping phenomenon. A calander month is on average 30.5 days long, while the time between full moons is 29.3 days. Normally, there is one full moon a month but, on a rare occasion, a month can have two full moons. There are different ways of determining which of the full moons is the blue one. Typically, a season has three full moons, however when one of the months has an extra full moon, the third full moon is condidered a blue moon. This is so rare, a blue moon will occur only seven times in 19 years, which works out to one every two to three years. Mark your calenders as the next one will occur on August 21, 2013.
Been reading 'The Field Guide to Natural Phenomena' by Keith Heidorn and Ian Whitelaw – so far it promises lots of interesting tidbits.
I've seen the moon turn a blood red – a frightening event if I didn't know why it changed colour. On that night it was a lunar eclipse. The earth had moved between the moon and sun, casting the moon into its shadow. Normally, the moon reflects sunlight directly from the sun making it a bright feature in the night sky. When the earth is in the way, the only sunlight to reach the moon is refracted around the earth and as a result the moon takes on a blood red colour (at least it did on the night I watched).
A blue moon is a completly different event, more of a bookkeeping phenomenon. A calander month is on average 30.5 days long, while the time between full moons is 29.3 days. Normally, there is one full moon a month but, on a rare occasion, a month can have two full moons. There are different ways of determining which of the full moons is the blue one. Typically, a season has three full moons, however when one of the months has an extra full moon, the third full moon is condidered a blue moon. This is so rare, a blue moon will occur only seven times in 19 years, which works out to one every two to three years. Mark your calenders as the next one will occur on August 21, 2013.
Been reading 'The Field Guide to Natural Phenomena' by Keith Heidorn and Ian Whitelaw – so far it promises lots of interesting tidbits.
Tuesday, December 7, 2010
What is it?
I was reading over a paper by John Peyssonel from the Royal Society's Philosophical Transactions (volume 50, 1757-1758, pages 585-589) titled 'Observations on the limax non cochleata purpur ferens, the naked snail producing purple.' It is about some sea creature and after reading it I have no clue what it is.
From the text (which is longest run on sentence I've ever seen):
Among the fish we meet with in the seas of the Antilles of America, we find, that this I am going to describe will appear precious, from the beautiful purple colour it produces, in the same manner, that the cuttle-fish produces its ink, if a means could be found to produce the liquor in a sufficient quantity to render it an article of commerce
The author goes on to describe this 'fish' as soft, viscous, without shells, scales or bones. It has no feet or fins. It acts like a slug when touched, in that it wreaths up as round as it can. In fact, they are so similar to snails and slugs the author calls them 'naked snails.' Their bodies are greenish in colour with black circular spots. They have two horns or antennae which might serve as eyes. Under a tough plate at the back of the body, it keeps a sack of purple juice. The purple juice can be deployed in defense just like a cuttle-fish uses it's ink.
Is this a description of a nudibranch? Or maybe a sea hare?
From the text (which is longest run on sentence I've ever seen):
Among the fish we meet with in the seas of the Antilles of America, we find, that this I am going to describe will appear precious, from the beautiful purple colour it produces, in the same manner, that the cuttle-fish produces its ink, if a means could be found to produce the liquor in a sufficient quantity to render it an article of commerce
The author goes on to describe this 'fish' as soft, viscous, without shells, scales or bones. It has no feet or fins. It acts like a slug when touched, in that it wreaths up as round as it can. In fact, they are so similar to snails and slugs the author calls them 'naked snails.' Their bodies are greenish in colour with black circular spots. They have two horns or antennae which might serve as eyes. Under a tough plate at the back of the body, it keeps a sack of purple juice. The purple juice can be deployed in defense just like a cuttle-fish uses it's ink.
Is this a description of a nudibranch? Or maybe a sea hare?
Monday, December 6, 2010
Where the tidal energy goes

The sun and moon put 3.5 TW of tidal power into the oceans, however, the total amount of energy present ocean-wide remains nearly constant from year to year. There must be a balance between the energy input and output, therefore tidal energy must go somewhere. The energy of the large-scale motions created by tides, along with energy from solar radiation and winds, is successively broken down to smaller scales and ultimately dissipated through viscous forces. This process doesn't happen evenly throughout the ocean, instead close proximity to structural hotspots such as continental shelves and sub-surface topography promote energy dissipation from basin-wide oscillations down to effects measured in millimeters. Complex internal flow dynamics are created at these hotspots which often support unique ecosystems.
Initially, tides are uniform with depth as the pull from the sun and moon act on the entire water column creating wavelengths on the order of thousands of kilometres. As the tide propagates it's molded by the shape of shorelines and roughness of the ocean floor. As it flows onto the shallower continental shelf, bottom friction slows the water down causing energy to accumulate in a smaller volume which acts to amplify the rise and fall of the water. Adding to the complexity of the dynamics, tidal flow over bottom topography produces internal tides (the discovery of which is an interesting tangent for another day). Shear instability, wave-wave interactions and topographical scattering all influence the rate of energy dissipation, and control whether internal tides dissipate near the generation site or far away. Ultimately, all tidal energy dissipates.
When the internal tide interacts with local existing internal waves, the kinetic energy is broken down to smaller scales. Waves are successively subdivided through non-linear processes and turbulence into smaller scale motion. Ultimately, a scale is reached that is small enough that viscosity dominates and the kinetic energy of the fluid motion is dissipated into heat. Effectively, there is an entire range of wave sizes that no longer directly receive energy from tides but are too large for viscosity effects to take hold; these mid-scale waves are called the 'internal subrange' (see my essay on turbulence for more info). In this range, the motion is completely determined by the rate energy enters at the large end of the scale and the rate it dissipates at the small end. In between, energy is transfered by inertial forces alone.
The 3.5 TW of tidal power is first dispersed into internal tides. Through wave-wave interactions and turbulence this energy is ultimately lost in small-scale diffusion. Turbulence is dissipative and irreversible by nature, resulting in kinetic energy lost to molecular viscosity acting on the smallest waves which reappears as thermal energy.
For more info:
Batchelor, G.K. (1953), The theory of homogeneous turbulence, 197pp.
Munk, W. and C. Wunsch (1998), Abyssal recipes II: energetics of tidal and wind mixing, Deep-Sea Research I, 45, 1977-2010.
St. Laurent, L., and C. Garrett (2002), The Role of Internal Tides in Mixing the Deep Ocean, J. Phys. Oceanogr, 32, 2882-2899.
Friday, December 3, 2010
Part 4: Blue Eyes
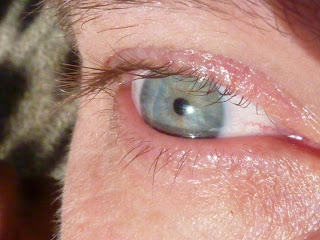
Here is part 4 of my 4 part series on nature's blues. Part 1 is here, part 2 is here and part 3 is here.
Gaining an understanding why something is the way it is in nature is not always a direct path. We know the blues found in nature are often the result of the object's internal structure rather than pigments, however the actual blue making process can vary. Although the blue of the sky and the blue of a feather can look like the same colour, the actual mechanism involved is very different. The two optical phenomenon involved in making these blues are Rayleigh scattering and coherent scattering. The blues produced either way can look the same.
So, which of these mechanisms is responsible for blue eyes? Rayleigh scattering is the culprit this time. Eyes appear blue when there are only small amounts of melanin present in the iris. Melanin is the pigment that makes the iris brown – a complete lack of melanin results in the pink eyes of an albino. When light passes into an minimally pigmented iris, tiny protein particles in the eye act just like the gas particles in the atmosphere blue wavelengths are preferentially scattered and the eyes appear blue.
As a tangent – here is how Leonardo da Vinci explained blue skies 200 years before Lord Rayleigh:
'I say that the blue which is seen in the atmosphere is not its own colour, but is caused by the heated moisture having evaporated into the most minute and imperceptible particles, which the beams of the solar rays attract and cause to seem luminous against the deep intense darkness of the region of fire that forms a covering among them.'
Monday, November 29, 2010
Part 3: Blue Feathers

Here is part 3 of my 4 part series on the scattered blues. Check out part 1 here and part 2 here.
Blue feathers have evolved in many species of birds. A blue jay's plumage is an excellent example with blue and white. You can see the black and blue of a Steller's jay in your own backyard. A male mountain bluebird has blue plumage of this type along with the head feathers of the male lazuli bunting; both can be found in central British Columbia. We know that feathers don't contain blue pigment, so the colour must be a result of the feather's structure.
In the late 1800s, just after the discovery of Rayleigh scattering, naturalists used this new concept to explain why blue feathers were blue. Since they didn't have the tools to examine the nanostructure (structure in the order of a billionth of a meter) of a feather, naturalists assumed that within the feather there existed transparent cells full of particles that were tiny enough to create Rayleigh scattering. Like the sky, blue light would be more efficiently scattered. These transparent cells would also contain pigments to absorb the longer wavelength colours. As a result, to our eyes these birds would appear blue.
Because Rayleigh scattering is incoherent, it produces the exact same colour irregardless of the observation direction. Since blue feathers in natural light don't change colour depending on what direction the naturalists looked at them, the assumption that their colour was formed through Rayleigh scattering seemed valid. But, in the 1930's, scientists examined a a non-iridescent blue feather under a directional light source. Colour variations were observed as the light source was moved – an iridescent characteristic that called into question the hypothesis of Rayleigh scattering making the feather blue.
By the 1940's, a cool new gadget came on the market – the electron microscope. Now naturalists could directly examine the internal nanostructure of blue feathers. Based on this first look, they interpreted the internal feather structure to contain randomly spaced objects. This meant scattered light would be incoherent leading giving support to the hypothesis of Rayleigh scattering. It took decades of further research to change this hypothesis and in the mean time many textbooks were written explaining that blue feathers were the result of Rayleigh scattering. By the 70's, scientists finally determined that the nanostructures were, in fact, not fully random. Instead they were a quasi-ordered matrix – not quite the perfect order of iridescence but not the full randomness required for Rayleigh scattering. Under natural light from all directions, like sunlight, these feathers appear to be the same colour from all directions. However when a directional light is shone on blue feathers the colour will change depending on the light direction.
Since the colour of a Steller's jay's feather comes from its internal structure on a tiny scale, a damaged feather would lose its blue colour. The dark pigments in the feather, that act to help show off the blue, would make damaged feather would look almost black. So if you are lucky enough to find a Steller's jay feather, take care of it.
Thanks to G. Hanke for the photo of mountain blue birds.
Saturday, November 27, 2010
Part 2: Blue Skies

Here is part 2 of my 4 part series on the scattered blues. Check out part 1 here.
On a sunny day, we perceive blue blanketing the sky, but, in reality, the sky has no colour. When traveling towards us, sunlight first hits earth's atmosphere. Earth's atmosphere is primarily composed of nitrogen (78%) and oxygen (21%) with bits of dust, water vapour and some inert argon, among other things. Water vapour and dust are the physically biggest components of the atmosphere, and are relatively large compared to the wavelengths of light. When light hits the water vapour and dust, is reflected in different directions, but the light remains white. So why does the sky appear blue?
In 1810, Goethe gave this explanation: “If the darkness of infinite space is seen through atmospheric vapours illuminated by the daylight, the blue colour appears.” His theory said colour comes from something within the atmosphere during the light of day. About the same time a more scientific inquiry was being made into the nature of scattering light. John Tyndall showed in an 1869 lab experiment that the blue hues of the sky could be created when white light was scattered by tiny particles. A few years later in 1871, John William Strutt, also known as Lord Rayleigh, was the first to describe the actual mechanism that makes the sky appear blue was a result of the tiny gas molecules of the atmosphere instead of the larger dust and water vapour.
When light collides with a gas molecule the results are different than when light hits a relatively large dust particle. Gas molecules are tiny compared to the wavelengths of light – several thousand times smaller. When light strikes a molecule, that molecule absorbs a specific wavelength (or colour) of the light's energy and later re-emits the same colour in all directions; a process called Rayleigh scattering. This type of scattering is an example of incoherent scattering. Lord Rayleigh discovered that molecules absorb energetic light (blues) at a much greater rate than less energetic light (reds).
Most of the longer wavelengths of light pass through our atmosphere unaffected, resulting in the full spectrum of sunlight with a higher ratio of blue wavelengths from the scattering. For this extra blue light to make the sky appear a brilliant blue, a dark background is required. Fortunately, beyond our atmosphere is the blackness of outer space, which makes an ideal dark background. The combined effect of the extra blue light and the black of outer space results in a sky that appears blue.
If you shift your gaze towards the horizon, the brilliant blues give way to paler colours and perhaps even white. The light reaching you from near the horizon passes through much more atmosphere, so the scattered blue light is scattered again and again, reducing its intensity. This is another consequence of Rayleigh scattering. Preferential scattering of blue light by our atmosphere occurs everywhere, not just above us. For example, light reflected from your hand to your eye is affected by this scattering, but the effect is so minuscule we can't detect it. Over a longer distance, like to a range of distant mountains, there is enough atmosphere to superimpose a blueish haze on our view of the mountains.
Friday, November 26, 2010
Part 1: Blue Skys and Blue Feathers – The Scattered Blues

A while back I wrote about why the sky was blue and why some feathers are blue (here) – well I didn't quite get it right, so I'm trying again. I've written a more detailed explanation which I'll post in four parts.
When I look around, I see lush greens of temperate rain forest, rich browns of fertile soil, lively yellows in fluttering butterflies, and luscious reds in ripe berries – but, not a lot of blues. If the sky is clear, it's the biggest blue object around, extending from horizon to horizon. Water reflects the blue of the sky, adding another layer of blue. On a lucky day, I'll catch a glimpse of a Steller's Jay showing off it's blue and black plumage, or a shimmering silver-blue dragon fly will dart by. I might even see a rare blue flower. On a gray winter day, the blue eyes of my favorite companion may be the only brilliant blue around. Other natural places have their own blue components, but in general, blues aren't common in nature. In fact, world-wide there just isn't a lot of natural blue pigments, thus the blues we see are often the result of optical properties within an object. These colours created as the result of an object's structure are called, creatively, 'structural colours'. Blue is a very common structural colour, and to understand why we'll need to start with some optics.
Sunlight is called 'white light' because it appears colourless. Within this colourless light lurks the full colour spectrum. Once, people thought white was the fundamental colour of light, and colours formed when something was added into the light. This theory was changed after the careful experimentation and observations of Sir Isaac Newton. Around 1670, Newton shone light through a prism creating a rainbow of hues on the other side. From this result, he concluded that white light contains all colours and that the prism simply separates them. Therefore, colour results from interactions between an object and light.
We now understand that white light is made up of tiny waves (which are simultaneously tiny particles if you want to add complexity). Light waves travel at the same speed but can have different wavelengths, that is, the distance between successive crests. Our brains perceive the different wavelengths as different colours. The longer wavelengths form reds, oranges and yellows, and the shorter wavelengths form greens, blues and violets. If you could watch waves of light pass by, more waves of blue would pass compared to waves of red – this means that the blue light has more energy. Light travels outward from its source, the sun, in a straight line until it collides with something. This collision could release all the hues in the spectrum or just a select few.
Scattering describes how light is diverted from its original straight path. Light scatters in two ways: coherent and incoherent. When scattering is coherent, spectacular effects such as iridescence can occur. Like a ball bouncing back from a flat wall, the light reflects precisely because the reflecting surface is geometrically regular. Similar colour light waves augment each other, further intensifying the effect. An iridescent feather's colour can change depending on viewing angle, a phenomenon easily observed in a Anna's Hummingbird gorget. Incoherent scattering resembles the result of throwing a rubber ball at a pole – it could bounce away in any direction. In this case, the scattering objects are randomly distributed relatively far apart. Scattering at one object occurs completely independently of the scattering at the other objects. Both coherent and incoherent scattering occur regularly in nature and can provide the mechanism for creating blue colours.
The photo is of a hyacinth macaw I took years ago at the San Diego Zoo.
Wednesday, November 24, 2010
Chaos

Back in the mid 90's, when I was on a winter army exercise, I was lent a copy of James Gleick's Chaos. We were in the middle of Alberta and it was cold – so cold we had moved our accommodations out of tents and into a heated H-hut (H-huts were built as temporary army barracks during WWII that were still in use). Our exercise was shut down until the temperature increased, so I had plenty of time to read.
I have always read about math and science. On one command post exercise, the brigade commander caught me reading during a lull in the action. In jest, he made a big deal of it. I suspect he thought I was reading a trashy romance novel, but instead I was reading a book on math. Shocked to discover the topic of my reading material, he told me that if math was what I was reading about then I was welcome to read on any of his exercises.
The book Chaos was the first time I had heard of chaos theory. I loved it. The fact that seemingly simple processes could generate such complexity was fascinating. Now I saw dripping faucets and swinging pendulums as gateways to observing chaos. To me, the most fascinating chaotic idea was turbulence. Water is complex! Move it just a bit and all sorts of phenomenon spring up including eddies and whorls.
Chaotic turbulent motions are found within the surface of stars, in combustion, in the ocean - even in water flowing from a faucet. Leonardo da Vinci included turbulence in his extensive studies, and he probably wasn't the first to study it. In all the centuries turbulence has been studied, we still haven't come up with a precise definition of what turbulence is. We know turbulence is what takes over when smooth fluid motion breaks into a complex network of eddy-like structures at all scales. Da Vinci's sketches of tiny eddies within small eddies within larger eddies and so on demonstrates the different size scales through which turbulent flow breaks up.
In Chaos, James Gleick describes turbulence this way: 'It is a mess of disorder at all scales, small eddies within large ones. It is unstable. It is highly dissipative, meaning that turbulence drains energy and creates drag. It is motion turned random.'
Okay, a picture of waves breaking on a beach isn't exactly a picture of turbulence, but it is the best I have.
Monday, November 22, 2010
It's snowing
It's snowing like mad out and very windy. In my backyard, the periodic wind gusts whip up the snowflakes into whirls and eddies producing momentary white outs. Fortunately, I don't have to go anywhere today. It doesn't snow often where I live, usually it remains green throughout the year. When it does snow it's like magic to me, the landscape is transformed from green to white like a idyllic Christmas card. The world seems hushed and new.
Today, the snow flakes are medium in size – not like our usual massive wet flakes that vanish once they hit the ground, or like the tiny prairie snowflakes that can be swept away with a broom. Each snowflake is a wonder of sharp-edged geometry. I don't know if each snowflake is truly unique or not – but I accept that it could be true. What I do know is that snow is just water and knowing this doesn't take away from the magic of a snow storm (if I'm inside).
Snow is just water – school children know this (hopefully, they also know about the perils of yellow snow). Winter outdoor survival manuals warn against eating snow directly only because it is cold – they just want you to melt it first.
I found a paper from 1673 on 'some observations touching the nature of snow' by N. Grew in the Philosophical Transactions of the Royal Society of London. He questions how snowflakes form and their geometry. He deduces that snowflakes come from icicles that form inside snow clouds. As they descend they thaw a bit, bump together and break apart eventually becoming the snowflakes we know.
For our modern view of how snowflakes form check out this.
Today, the snow flakes are medium in size – not like our usual massive wet flakes that vanish once they hit the ground, or like the tiny prairie snowflakes that can be swept away with a broom. Each snowflake is a wonder of sharp-edged geometry. I don't know if each snowflake is truly unique or not – but I accept that it could be true. What I do know is that snow is just water and knowing this doesn't take away from the magic of a snow storm (if I'm inside).
Snow is just water – school children know this (hopefully, they also know about the perils of yellow snow). Winter outdoor survival manuals warn against eating snow directly only because it is cold – they just want you to melt it first.
I found a paper from 1673 on 'some observations touching the nature of snow' by N. Grew in the Philosophical Transactions of the Royal Society of London. He questions how snowflakes form and their geometry. He deduces that snowflakes come from icicles that form inside snow clouds. As they descend they thaw a bit, bump together and break apart eventually becoming the snowflakes we know.
For our modern view of how snowflakes form check out this.
Thursday, November 18, 2010
An essay on turbulence
An essay of mine on one of my favorite topics (that is turbulence) has been published in my university grad journal. Check it out here.
Tuesday, November 16, 2010
A bloody green
Further to my post from yesterday, I stumbled across an interesting article from the 1818 edition of the Philosophical Transactions of the Royal Society of London, volume 108, pages 110 to 117. The paper was called 'A Few Facts Relative to the Colouring Matters of Some Vegetation' by James Smithson – an interesting person that I'm planning on digging up more information on.
I found this buried at the end in a section called 'Some Animal Greens':
There are small gnats of a green colour: crushed on paper, they make a green stain, which is permanent.
This brings to mind a child squishing bugs to see what colour their insides are. I've found no other reference to green dyes from gnats, so I'm assuming crushing gnats didn't make it into commercial production.
I found this buried at the end in a section called 'Some Animal Greens':
There are small gnats of a green colour: crushed on paper, they make a green stain, which is permanent.
This brings to mind a child squishing bugs to see what colour their insides are. I've found no other reference to green dyes from gnats, so I'm assuming crushing gnats didn't make it into commercial production.
Monday, November 15, 2010
Actual bloody colours
I've been thinking about my bloody colours post of a little while back. Magenta and solferno were named because they reminded observers of the after effects of a battle. What about colours made from actual blood? That is, by killing a critter. These dyes exist, and some are still in use today.
Fantastic reds can be made from crushed insects. I wonder who was the first who thought of grinding up dried bugs to dye cloth? One of these dyes is Kermes, an ancient dye extracted from an insect (Coccus ilicis) that resided in the middle east. This bug lives as a parasite on oaks, producing carminic acid (the base component for a dye) to deter predators. Kermes is the root of the word crimson and predictably, cloth dyed with kermes turns out a bluish-red. Skilled dyers could even produce a scarlet cloth. About 70,000 insects are needed to make only one pound of dye – making a very bloody dye.
Cochineal, also known as carmine, is another ancient bloody dye produced from similar insect (Dactylopius coccus). This bug resides on cactus in Mexico and has been the foundation of a red dye for millennium. This dye is chemically the same as kermes except ten times stronger – less of them needed to die to produce the same amount of dye. When the Spanish brought this dye back to Europe in the mid 1500s, it quickly over shadowed kermes because it was a cheaper alternative. Both kermes and cochineal have been widely used to colour foods dating back to the middle ages, and cochineal is still in use now. As a food colourant it's called by many names, including 'natural red 4'.
Throughout the ages other similar insects have been used to make red dyes. Polish cochineal (Porphyrophora polonica), a insect that lives on the roots of herbs in Poland, was once used to make reds as an alternative to kermes. In India, a red dye was made from a secretion left behind by an insect in the same family (Laccifer lacca), I think the bug got to live in this case – but, I don't know for sure. In South East Asia, reds called lac, could be made from a whole family of related insects, which also provided the foundation for shellac (often used as a protective coat for wood).
Tyrian purple held the title of the most prestigious dye in antiquity. In Roman times if you were caught wearing clothing dyed this purple and weren't royalty it was considered a crime, of course affording this colour if you weren't royalty was virtually impossible. The complex technique for making this dye was discovered around 1500BC by the Phoenicians, an ancient Mediterranean seafaring traders. Tyrian purple is made from a pale yellow mucous secretion from some molluscs, commonly known as sea snails. It is possible to 'milk' these snails, in which case, they wouldn't be harmed – however, this is labour intensive so more destructive methods were used. Often the snails would simply be crushed to get their secretion. From one source, the snails were salted and left for three days to extract the liquid. The liquid was boiled for ten days after which fibers would be soaked in the resulting liquid for five hours. Finally, the resulting fabric would have to be exposed to sunlight where it changed colour from deep yellow, through green and blue to finally purple.
To dye a metre of cloth, 12,000 molluscs would be required (ie killed). Since they were making luxury fabrics, often a fabric would be dyed more than once to get the best shade. Different snails gave different shades, to get the best purple cloth would be first dyed in one species of snails then in another. Fortunately for the snails, synthetic dyes have completely replaced the original tyrian purple.
For more info check here, including some nice pictures.
Fantastic reds can be made from crushed insects. I wonder who was the first who thought of grinding up dried bugs to dye cloth? One of these dyes is Kermes, an ancient dye extracted from an insect (Coccus ilicis) that resided in the middle east. This bug lives as a parasite on oaks, producing carminic acid (the base component for a dye) to deter predators. Kermes is the root of the word crimson and predictably, cloth dyed with kermes turns out a bluish-red. Skilled dyers could even produce a scarlet cloth. About 70,000 insects are needed to make only one pound of dye – making a very bloody dye.
Cochineal, also known as carmine, is another ancient bloody dye produced from similar insect (Dactylopius coccus). This bug resides on cactus in Mexico and has been the foundation of a red dye for millennium. This dye is chemically the same as kermes except ten times stronger – less of them needed to die to produce the same amount of dye. When the Spanish brought this dye back to Europe in the mid 1500s, it quickly over shadowed kermes because it was a cheaper alternative. Both kermes and cochineal have been widely used to colour foods dating back to the middle ages, and cochineal is still in use now. As a food colourant it's called by many names, including 'natural red 4'.
Throughout the ages other similar insects have been used to make red dyes. Polish cochineal (Porphyrophora polonica), a insect that lives on the roots of herbs in Poland, was once used to make reds as an alternative to kermes. In India, a red dye was made from a secretion left behind by an insect in the same family (Laccifer lacca), I think the bug got to live in this case – but, I don't know for sure. In South East Asia, reds called lac, could be made from a whole family of related insects, which also provided the foundation for shellac (often used as a protective coat for wood).
Tyrian purple held the title of the most prestigious dye in antiquity. In Roman times if you were caught wearing clothing dyed this purple and weren't royalty it was considered a crime, of course affording this colour if you weren't royalty was virtually impossible. The complex technique for making this dye was discovered around 1500BC by the Phoenicians, an ancient Mediterranean seafaring traders. Tyrian purple is made from a pale yellow mucous secretion from some molluscs, commonly known as sea snails. It is possible to 'milk' these snails, in which case, they wouldn't be harmed – however, this is labour intensive so more destructive methods were used. Often the snails would simply be crushed to get their secretion. From one source, the snails were salted and left for three days to extract the liquid. The liquid was boiled for ten days after which fibers would be soaked in the resulting liquid for five hours. Finally, the resulting fabric would have to be exposed to sunlight where it changed colour from deep yellow, through green and blue to finally purple.
To dye a metre of cloth, 12,000 molluscs would be required (ie killed). Since they were making luxury fabrics, often a fabric would be dyed more than once to get the best shade. Different snails gave different shades, to get the best purple cloth would be first dyed in one species of snails then in another. Fortunately for the snails, synthetic dyes have completely replaced the original tyrian purple.
For more info check here, including some nice pictures.
Tuesday, November 9, 2010
A recycled documentary idea
Every couple of years, when I'm channel surfing, I stumble across a new documentary on what happened to the Amber Room. By the way, it's still lost. The Amber Room was built for a palace near St. Petersburg of gold leaf, mirrors and amber. The shiny yellows and golds are ornately detailed for an aristocratic taste found in another era. The room's complex construction took about eight years from 1701 to 1709 and included six tones of amber. In WWII, the room was covered in wallpaper in an attempt to prevent looting. It didn't work. The room was crated up by the Nazi's and shipped off. Although there has been repeated announcements of imminent discovery and theories on its fate, the Amber Room has yet to be recovered (it has however been reconstructed).
Along the Baltic Sea, pieces of amber wash ashore and have been collected since the stone age. The Dominion Republic is also a great place to find amber. In fact, amber is quite common and found all over the globe. But, what is amber? According to Wikipedia, it's fossilized tree resin. Coniferous trees are big producers of resins which is a hydrocarbon secretion. If you have ever handled a chunk of fir or pine tree, the turpentine like smell from the sticky residue left on your hands is the resin. Varnishes, adhesives, incense and perfumes have all been made from resins throughout the eons. Resin is different than sap, as sap is the fluid that transports nutrients around a plant (maple syrup is an example of a product made from sap).
Most amber is a warm yellow to orange brown. It's known to range in colour from a pale lemon yellow to red. Rare blue amber is formed when pyrites are included. Amber is often considered a gem stone, although it's not indestructible like a diamond.
To me, the most interesting thing about amber is that it can provide a window into ancient worlds. The oldest amber found so far is 320 million years old. We can learn about trees that have since gone extinct from the amber they produced. Since, resins are sticky stuff, the fossilized version ends up with all sorts of interesting things in it. Pollen from when our climate was different can be pulled out of amber giving us clues about ancient conditions.
The most spectacular are the trapped critters – insects, spiders, frogs and lizards. One misstep into the resin and these creatures are forever trapped. Baltic amber has few critters while Dominican Republic amber has more. Over the last few years scientists have isolated DNA from trapped termites, bees and butterflies. A great amber deposit with insects was recently found in India which promises more interesting finds. However, the Jurassic Park concept of isolating dinosaur DNA from the mosquito that bit it, is still fiction.
As a side note: amber ale has the deep yellow-orange of stereotypical amber, thus the name (no actual amber is involved in making amber ale).
Along the Baltic Sea, pieces of amber wash ashore and have been collected since the stone age. The Dominion Republic is also a great place to find amber. In fact, amber is quite common and found all over the globe. But, what is amber? According to Wikipedia, it's fossilized tree resin. Coniferous trees are big producers of resins which is a hydrocarbon secretion. If you have ever handled a chunk of fir or pine tree, the turpentine like smell from the sticky residue left on your hands is the resin. Varnishes, adhesives, incense and perfumes have all been made from resins throughout the eons. Resin is different than sap, as sap is the fluid that transports nutrients around a plant (maple syrup is an example of a product made from sap).
Most amber is a warm yellow to orange brown. It's known to range in colour from a pale lemon yellow to red. Rare blue amber is formed when pyrites are included. Amber is often considered a gem stone, although it's not indestructible like a diamond.
To me, the most interesting thing about amber is that it can provide a window into ancient worlds. The oldest amber found so far is 320 million years old. We can learn about trees that have since gone extinct from the amber they produced. Since, resins are sticky stuff, the fossilized version ends up with all sorts of interesting things in it. Pollen from when our climate was different can be pulled out of amber giving us clues about ancient conditions.
The most spectacular are the trapped critters – insects, spiders, frogs and lizards. One misstep into the resin and these creatures are forever trapped. Baltic amber has few critters while Dominican Republic amber has more. Over the last few years scientists have isolated DNA from trapped termites, bees and butterflies. A great amber deposit with insects was recently found in India which promises more interesting finds. However, the Jurassic Park concept of isolating dinosaur DNA from the mosquito that bit it, is still fiction.
As a side note: amber ale has the deep yellow-orange of stereotypical amber, thus the name (no actual amber is involved in making amber ale).
Thursday, November 4, 2010
More than iron

I tend to be low in iron and periodically have to take iron supplements. Right now I have the supplement my doctor recommended to me and I've made the mistake of looking at the ingredients. After the iron (which I'm okay with because it is the point of taking these) there is: D&C yellow no. 10, FD&C red no. 2, FD&C red no. 3, FD&C yellow no. 6, gelatin, lactose, povidone, silicon dioxide, sodium lauryl sulphate, sucrose, talc and titanium dioxide. All I wanted was some iron, instead I'm getting a hockey sock full of chemicals. I'm also confused why my iron pills have to be a bright red.
D&C yellow no. 10 (quinoline yellow) is derived from petroleum or coal tar. It has regulated minimums of lead, arsenic, mercury and cadmium, which scares me a bit. FD&C red no. 2 (amaranth) is another coal tar derived dye that is actually banned in the the United States because it is a carcinogen. FD&C red no. 3 (erythrosine) is the same dye dentists use to demonstrate how much plaque are on your teeth. FD&C yellow no. 6 is a lemon yellow dye that may cause everything from hives to kidney tumors.
More adverse reactions of inactive ingredients can be found here.
The gelatin is likely needed to make the outer capsule – so I'm okay with that. Lactose is a sugar found in milk, plenty of people can't tolerate it, so why include it? Povidone is a binder which is also used in glue sticks, I don't know if it is bad or not. Silicon dioxide is probably harmless. Sodium lauryl sulphate is highly effective in getting oil stains out. Sucrose, also known as table sugar, isn't too bad. Talc is added as a glidant, which I assume is to help the pill go down or make the manufacturing process smoother. Titanium dioxide is a white pigment that has also been used on rockets and as a sunscreen.
These 'non-medicinal' items may not be dangerous in the amount the pills contain, but I just don't think I need them. I'm going to find an iron supplement without all these questionable ingredients.
Thursday, October 28, 2010
Twilight - the space between day and night (without vampires)

How many painters have attempted to capture the gradiated hues of a sunset in pigments? It takes true mastery to get translucent fleeting colours from flat pigments; some artists do it exceptionally well, but most don't. Detailed observations of actual sunsets is the key: what colours go where? How do a few clouds change things? Cameras can capture some aspects of a sunset, but often miss the nuances. With my digital camera, I took this picture near the end of my drive to Winnipeg last summer – the sunset was much more stunning in person. However, nothing beats sitting on a patio somewhere with a view (perhaps with an accompanying beverage) and watching day turn to night.
Sunsets are a spectacular end to the day – however the entire process of shifting from day to night is called twilight. According to a book published in 1966 by Georgii Rozenberg (called 'Twilight' without a single mention of vampires – I like to read old science books): The term twilight refers to the entire complex of optical phenomenon that take place in the atmosphere when the sun is near the horizon. It occupies the interval separating daytime conditions of illumination from night.
I live far enough north to get reasonably extended twilights. The downside is that I live far enough north that twilight can start in the late afternoon on the shorter days of the year. Every twilight is unique and the shift from day time brilliance to more subdued hues feels almost magical. During twilight, the illumination at the ground decreases by a factor of a billion. If seen from space, twilight covers a global swath separating day from night. Twilight happens because the earth is rotating – so it will occur on every rotating planet with an atmosphere.
Looking up at the sky has been a pass-time for eons. However, early in the 21st century, before spaceflight was common, a keen interest in studying twilight emerged to provide details about the composition of the atmosphere – useful to know if you are trying to communicate by radio.
Many interesting phenomenon occur each twilight (I'll write more in other posts), however sunsets are the most obvious. Atmospheric optical properties are responsible for the vivid colours of sunset. Specifically, the amount of water vapour and dust play a huge role. In 1863, atmospheric scattering and attenuation of light were shown to produce the sunset colours. Since entire books have been written on sunsets, so my description will be brief.
When the sun drops towards the horizon, the sunlight must pass through more atmosphere. Since shorter wavelengths of light are scattered preferentially (see Rayleigh scattering post), the sun appears in redder tones (red is at the long end of the colour spectrum) and the near-by sky takes on yellow and orange hues. When the sun is about 5 degrees below the horizon (like in my picture above), it is out of sight to an observer on the ground. The sky above the horizon remains brightly coloured in deep reds while mountain tops and clouds are bathed with crimson and purple light.
A cool home experiment for generating a sunset in a glass can be found here.
Monday, October 25, 2010
Bloody colours
To a bloody war and sickly season - the traditional Thursday toast of the British Navy.
Since I've already written about blue (here and here), a friend suggested I write a post on redder colours, specifically ones named after bloody battles. I only found two: magenta and solferino – both are purplish red colours, perhaps even the same colour. Magenta and Solferino are both towns in Northern Italy that were caught up in the second Italian war of Independence at the same time synthetic dyes were being made from coal tar for the first time. Magenta as a colour name is still in common use, while Solferino was the more important battle. A witness to the battle of Solferino, Henry Dunant, found it so horrible he began a campaign that ultimately resulted in the founding of the Red Cross.
In 1859, Emmanuel Verguin's experiments with aniline dyes (ie the ones from coal tar) resulted in a rich crimson red. He called the colour fuchsine after the fuchsia flower and it was an instant hit. This was a prominent colour of the uniforms at both the battle of Magenta and Solferino, both in June 1859, so I don't know if the colour took these names because of the uniforms or the bloodiness of the battlefields (I've found references both ways). A few years later, the colour's name was once more changed, this time to rosaniline, but magenta is the name that stuck. A arsenic acid oxidation process was required to make this dye causing some of its wearers to be poisoned – leaving magenta even more bloody. (For more details of synthetic dyes 'Mauve' by Simon Garfield is a good read)
If you took a good look at the colour spectrum of light, magenta wouldn't be found. Magenta is considered an extra-spectral colour because it cannot be generated by a single wavelength of light. It is formed in our minds when there are equal parts of blue and red light (in truth colours only exist because our brains perceive them).
Since I've already written about blue (here and here), a friend suggested I write a post on redder colours, specifically ones named after bloody battles. I only found two: magenta and solferino – both are purplish red colours, perhaps even the same colour. Magenta and Solferino are both towns in Northern Italy that were caught up in the second Italian war of Independence at the same time synthetic dyes were being made from coal tar for the first time. Magenta as a colour name is still in common use, while Solferino was the more important battle. A witness to the battle of Solferino, Henry Dunant, found it so horrible he began a campaign that ultimately resulted in the founding of the Red Cross.
In 1859, Emmanuel Verguin's experiments with aniline dyes (ie the ones from coal tar) resulted in a rich crimson red. He called the colour fuchsine after the fuchsia flower and it was an instant hit. This was a prominent colour of the uniforms at both the battle of Magenta and Solferino, both in June 1859, so I don't know if the colour took these names because of the uniforms or the bloodiness of the battlefields (I've found references both ways). A few years later, the colour's name was once more changed, this time to rosaniline, but magenta is the name that stuck. A arsenic acid oxidation process was required to make this dye causing some of its wearers to be poisoned – leaving magenta even more bloody. (For more details of synthetic dyes 'Mauve' by Simon Garfield is a good read)
If you took a good look at the colour spectrum of light, magenta wouldn't be found. Magenta is considered an extra-spectral colour because it cannot be generated by a single wavelength of light. It is formed in our minds when there are equal parts of blue and red light (in truth colours only exist because our brains perceive them).
Wednesday, October 20, 2010
A beach
My favorite beach is one I used to go to when I was a kid. Not a traditional sandy expanse, this beach was far enough off the beaten path that it was generally deserted – an ideal location for my imaginary world to thrive.
To get to the beach I would walk down a blackberry lined sandy road where the lapping of waves and calling of seabirds would reach my ears long before they came into view. Once I reached the end of the road a small path was cut into the broom and blackberries. I would navigate this steep path down a short cliff. From there, I could see the undulating surface of the sea extending in all directions until it was broken by a blue-toned landscape a long distance off. Huge driftwood pieces, tossed ashore by winter storms, now blocked the path. I remember balancing along these logs until I reached a field of tiny stones. Beyond the stones was my favorite part: a wide band of sandstone formations. Unless it was the lowest of low tides, beyond the sandstone lapped the waves. When there was an extreme low tide, a new landscape of seaweed covered rocks and tide pools was exposed.
The sea-worn sandstone captured my imagination the most. Each shape could be an fantastical dimpled animal large enough to sit on and I could ride the dusty yellow beast with sandpaper textured skin anywhere my imagination could devise. Or the shapes could be mushroom formations rising up from a pool of lava, forcing me to leap between each one to get to the other side. Or the shapes could be worn down fortifications of an ancient castle, giving me a place to hide from my enemies. Some shapes had dimpled pickets that held treasures like tiny shells and stones. In some places shallow pools that were warm enough to wade in separated the shapes. These pools would be home to seaweeds, bullheads (sculpins), crabs and snails; the pools that only existed at low tide housed anemones and starfish.
The maze of rough sandstone shapes could entertain me for hours – and held different objects to discover every time I was there. Some days abandoned fishing floats would wash up, other days there would be uniquely shaped driftwood that could be imagined as tridents, swords or crutches. Always there would be seabirds watching from a safe distance and cackling amongst themselves.
I've never seen another beach with the same type of sandstone formations as the one from my childhood. That childhood beach actually exists, but I haven't been there for years. I've gone back to other places that held magic for me as a child, but now they are just places, no longer manifestations of my imagination. So I won't physically visit that beach of my childhood, instead I'll just visit the memories.
To get to the beach I would walk down a blackberry lined sandy road where the lapping of waves and calling of seabirds would reach my ears long before they came into view. Once I reached the end of the road a small path was cut into the broom and blackberries. I would navigate this steep path down a short cliff. From there, I could see the undulating surface of the sea extending in all directions until it was broken by a blue-toned landscape a long distance off. Huge driftwood pieces, tossed ashore by winter storms, now blocked the path. I remember balancing along these logs until I reached a field of tiny stones. Beyond the stones was my favorite part: a wide band of sandstone formations. Unless it was the lowest of low tides, beyond the sandstone lapped the waves. When there was an extreme low tide, a new landscape of seaweed covered rocks and tide pools was exposed.
The sea-worn sandstone captured my imagination the most. Each shape could be an fantastical dimpled animal large enough to sit on and I could ride the dusty yellow beast with sandpaper textured skin anywhere my imagination could devise. Or the shapes could be mushroom formations rising up from a pool of lava, forcing me to leap between each one to get to the other side. Or the shapes could be worn down fortifications of an ancient castle, giving me a place to hide from my enemies. Some shapes had dimpled pickets that held treasures like tiny shells and stones. In some places shallow pools that were warm enough to wade in separated the shapes. These pools would be home to seaweeds, bullheads (sculpins), crabs and snails; the pools that only existed at low tide housed anemones and starfish.
The maze of rough sandstone shapes could entertain me for hours – and held different objects to discover every time I was there. Some days abandoned fishing floats would wash up, other days there would be uniquely shaped driftwood that could be imagined as tridents, swords or crutches. Always there would be seabirds watching from a safe distance and cackling amongst themselves.
I've never seen another beach with the same type of sandstone formations as the one from my childhood. That childhood beach actually exists, but I haven't been there for years. I've gone back to other places that held magic for me as a child, but now they are just places, no longer manifestations of my imagination. So I won't physically visit that beach of my childhood, instead I'll just visit the memories.
Friday, October 15, 2010
Ships of the desert
Years ago I wrote a story about a woman crossing the Sahara Desert. Part of the reason I put her there was my discovery that native desert folk could look at a camel's footprint and tell its gender. I have no idea if this is true because I've never been to the Sahara, or anywhere where there were people identifying a camel's gender from footprints. To be honest, the closest I've been to a camel is a zoo (the warning about spitting sign ensured I didn't even get close to the fence). According to the reference I found years ago (and can't find now), camels of different genders were used for different purposes, so the pads of their feet would wear differently.
The idea of camels as 'ships of the desert' brings to my mind lines of camels carrying exotic goods (in my mind each camel is swarming with their 'horse fly' equivalent). Like every school kid knows, a one humped camel is a dromedary and a two humped camel is a bactrian; I'm thinking about the dromedary camel. These camels originated in Arabia where they were domesticated over 4000 years ago. From there people took them to North Africa, India, Pakistan and Australia – then even further afield as I've seen them in North American zoos.
A camel can carry up to 600 lbs of freight up to a distance of 160km a day (a fact I found in a children's book), making them outstanding pack animals already evolved for a desert environment. They can be speedy; racing camels can get up to 33 km/hr for a 10 km race. Back to their footprints: they have broad feet that are heavily padded to allow them to walk stably over hot sand. I guess if there is some humidity, they could make a pretty distinctive footprint. But, if someone told me they could tell the gender of the camel from that footprint, I would assume they were pulling my leg.
The idea of camels as 'ships of the desert' brings to my mind lines of camels carrying exotic goods (in my mind each camel is swarming with their 'horse fly' equivalent). Like every school kid knows, a one humped camel is a dromedary and a two humped camel is a bactrian; I'm thinking about the dromedary camel. These camels originated in Arabia where they were domesticated over 4000 years ago. From there people took them to North Africa, India, Pakistan and Australia – then even further afield as I've seen them in North American zoos.
A camel can carry up to 600 lbs of freight up to a distance of 160km a day (a fact I found in a children's book), making them outstanding pack animals already evolved for a desert environment. They can be speedy; racing camels can get up to 33 km/hr for a 10 km race. Back to their footprints: they have broad feet that are heavily padded to allow them to walk stably over hot sand. I guess if there is some humidity, they could make a pretty distinctive footprint. But, if someone told me they could tell the gender of the camel from that footprint, I would assume they were pulling my leg.
Thursday, October 14, 2010
Black jackets and being seen – or "How Not To Be Seen" for Monty Python fans
I recently bought a new jacket because my old one fell apart. I wanted a bright colour, easily seen by traffic when I walk to work on rainy, gray days (for obvious reasons). I also wanted my new jacket to be waterproof, again for those rainy days. I'm not much of a shopper, but I did shop around and the only jacket that I could find that met my criteria was black. So now I have a black jacket – the exact wrong colour for high visibility. As a teenager, I had a khaki jacket (army surplus) – a colour designed to blend into wilderness surroundings. I'd often wear this jacket camping. To be seen, I wore nuclear orange gloves that my grandmother had given me. From a distance often all that could be seen of me were the gloves. I actually loved the juxtaposition of my khaki jacket and nuclear gloves. Which brings me to how things appear to "stand out", using sharp contrasts like my gloves.
Distinctive shapes also stand out. For example, our brains are hard-wired to see faces, even in bizarre places like stucco walls and clouds. For this reason, soldiers often paint disruptive green patterns across their faces when they want to vanish in the woods. Straight lines where they shouldn't be also stick out as nature generally doesn't have straight edges. Ever looked at a satellite photo of a wilderness area and had the square shape of a cabin pop out? Movement sticks out. I can find escaped crickets (we keep critters that eat crickets) on out cricket coloured carpets because they move. So, if you are hiding from bad guys: stay still.
On my walk to work I want to be seen. My nuclear orange gloves vanished years ago so I can't rely on them. A retroreflector is an option which is just a good reflector set up to bounce the light right back where it came from irregardless of orientation. The shine from a cat's eye when light hits it is an example. One type of retroreflector is a corner reflector, which is three mirrors put together like the inside corner of a cube. Since many small versions of retroreflectors can be put together as a thin sheet and attached to a fabric, clothing can be made from them. On a dark, rainy day I could wear a retroreflector band around my wrist which would bounce the light of car headlights back towards the driver, warning the driver of my presence. So my problem is solved, I need to find retroreflector wrist bands: but where else are retroreflectors used?
Retroreflectors have made their way to the moon both on American (Apollo 11, 14 and 15) and Russian (Lunakhod 1 and 2) spacecraft as a way to determine the distance between the earth and moon. This is done by aiming a laser on earth at the retroreflector and measuring how long the light takes to return back. This method has found the average distance from the earth to the moon is about 385,000 km. All the retroreflectors on the moon are still in use. They are the only Apollo experiment still returning data from the moon (I don't know if there is Russian gear other than the retroreflectors still transmitting, but I doubt it) and has resulted in and improved knowledge of the moon's orbit.
On a tangent... somehow the Russian Lunokhod 1 rover got lost. On November 17, 1970, Luna 17 arrived at the moon and released the Lunokhod 1 rover to explore. This rover trundled over 10 km, taking samples of the lunar surface and transmitting pictures, until its power ran out at year later. Since a retroreflector was mounted on the rover, scientists were able to keep track of it with lasers from earth until 1974. Then they lost track of it (not sure why because it was no longer moving). Recently, NASA's Lunar Reconnaissance Orbiter spotted the rover's tracks (remember, the only movement on the moon has been us and there is no wind to cover tracks) and was able to pinpoint the rover's location. On 22 April 2010, a laser was bounced of its retroreflector once again.
So a retroreflector turns out to be an excellent way to be seen even from really far away. However, if you are really good with your optics, a retroreflector can be set up that will render one almost invisible.
Distinctive shapes also stand out. For example, our brains are hard-wired to see faces, even in bizarre places like stucco walls and clouds. For this reason, soldiers often paint disruptive green patterns across their faces when they want to vanish in the woods. Straight lines where they shouldn't be also stick out as nature generally doesn't have straight edges. Ever looked at a satellite photo of a wilderness area and had the square shape of a cabin pop out? Movement sticks out. I can find escaped crickets (we keep critters that eat crickets) on out cricket coloured carpets because they move. So, if you are hiding from bad guys: stay still.
On my walk to work I want to be seen. My nuclear orange gloves vanished years ago so I can't rely on them. A retroreflector is an option which is just a good reflector set up to bounce the light right back where it came from irregardless of orientation. The shine from a cat's eye when light hits it is an example. One type of retroreflector is a corner reflector, which is three mirrors put together like the inside corner of a cube. Since many small versions of retroreflectors can be put together as a thin sheet and attached to a fabric, clothing can be made from them. On a dark, rainy day I could wear a retroreflector band around my wrist which would bounce the light of car headlights back towards the driver, warning the driver of my presence. So my problem is solved, I need to find retroreflector wrist bands: but where else are retroreflectors used?
Retroreflectors have made their way to the moon both on American (Apollo 11, 14 and 15) and Russian (Lunakhod 1 and 2) spacecraft as a way to determine the distance between the earth and moon. This is done by aiming a laser on earth at the retroreflector and measuring how long the light takes to return back. This method has found the average distance from the earth to the moon is about 385,000 km. All the retroreflectors on the moon are still in use. They are the only Apollo experiment still returning data from the moon (I don't know if there is Russian gear other than the retroreflectors still transmitting, but I doubt it) and has resulted in and improved knowledge of the moon's orbit.
On a tangent... somehow the Russian Lunokhod 1 rover got lost. On November 17, 1970, Luna 17 arrived at the moon and released the Lunokhod 1 rover to explore. This rover trundled over 10 km, taking samples of the lunar surface and transmitting pictures, until its power ran out at year later. Since a retroreflector was mounted on the rover, scientists were able to keep track of it with lasers from earth until 1974. Then they lost track of it (not sure why because it was no longer moving). Recently, NASA's Lunar Reconnaissance Orbiter spotted the rover's tracks (remember, the only movement on the moon has been us and there is no wind to cover tracks) and was able to pinpoint the rover's location. On 22 April 2010, a laser was bounced of its retroreflector once again.
So a retroreflector turns out to be an excellent way to be seen even from really far away. However, if you are really good with your optics, a retroreflector can be set up that will render one almost invisible.
Thursday, October 7, 2010
Sea foam – and why it can be bad for birds
The word “sea-foam” has a feminine mystique to it. I picture it used for frilly prom dresses and girly princess rooms. In the real world, sea foam is never the pretty light aqua colour of paint chips, instead it looks like dirty cappuccino foam – a yellowed off-white often with chunks of stuff in it. As the sea sloshes, salts, chemicals, dead plants, decomposing fish and sea weeds are churned into sea foam. Individual bubbles link together, and when a surface wave passes these bubbles they mass together as they swirl upwards to make foam on the sea surface.
Sea foam on the water's surface changes how wind energy is transferred into the water. This transfer of energy is a type of friction, called the wind stress, and it can play a part in important oceanographic processes such as currents and upwelling. What sea foam does is add another layer between the wind and ocean, so instead of the wind pushing the water, the wind has to push the foam and then the foam pushes the water, which is a much less efficient process. Sea foam can do much worse things.
In the coastal ocean red tides occur, which is a dangerous form of algal bloom. A red tide is better described as a Harmful Algal Bloom or HABs, because HABs aren't all red and not all red tides are HABs. Specific phytoplankton (little water plants) full of toxins are the culprit and are bad news for shell fish and anything that eats them, like us. Recently, migrating birds along the Washington State coast were found dead in large numbers at the same time as an HAB occurred. The birds weren't poisoned; instead they were freezing to death. Why? One or more of the HABs causing phytoplankton were getting churned up into the sea foam. Birds would rummage around in this foam getting themselves covered in it, because normally sea foam isn't a danger to them. But in this case, the phytoplankton-laced foam coated the bird's feathers is such a way that they lost waterproofing and insulation, so that the poor birds froze.
Sea foam on the water's surface changes how wind energy is transferred into the water. This transfer of energy is a type of friction, called the wind stress, and it can play a part in important oceanographic processes such as currents and upwelling. What sea foam does is add another layer between the wind and ocean, so instead of the wind pushing the water, the wind has to push the foam and then the foam pushes the water, which is a much less efficient process. Sea foam can do much worse things.
In the coastal ocean red tides occur, which is a dangerous form of algal bloom. A red tide is better described as a Harmful Algal Bloom or HABs, because HABs aren't all red and not all red tides are HABs. Specific phytoplankton (little water plants) full of toxins are the culprit and are bad news for shell fish and anything that eats them, like us. Recently, migrating birds along the Washington State coast were found dead in large numbers at the same time as an HAB occurred. The birds weren't poisoned; instead they were freezing to death. Why? One or more of the HABs causing phytoplankton were getting churned up into the sea foam. Birds would rummage around in this foam getting themselves covered in it, because normally sea foam isn't a danger to them. But in this case, the phytoplankton-laced foam coated the bird's feathers is such a way that they lost waterproofing and insulation, so that the poor birds froze.
Tuesday, October 5, 2010
Foamy frothy fun
Most of my days end with a long soak in a deliciously hot bubble bath. I don't need fancy scents, instead I enjoy the heat of the water and playful texture of the bubbles. Bubbles rise up as a mound ringing the stream of water from the faucet. Further away, bubbles slide into irregular shapes reminiscent of fictitious moon bases and futuristic homes. If it's really quiet, the muffled pops as multiple bubbles end their existence is audible. In addition to a relaxing end to a day, my bubble bath is an example of a foam.
Foams form when billions of tiny bubbles are packed together within a solid or a liquid. Irregular sized bubbles are common in all foams except the most idealized ones. Like what happens for individual bubbles (check out my bubble post), it's surface tension that helps keep a foam stable. Liquid foams break down eventually – there are even chemicals on the market to make this process go faster. Gas can diffuse from small bubbles into large ones and eventually out of the foam, or gravity can drain liquid out the bottom making bubbles so weak they pop on top.
A Belgian physicist, Joseph Plateau (1801-1883), figured out the basis of what we know about soap films and foams. His diverse interests also included spending time with moving image illusions like the action one sees when using a "flip-book". Back to soap film, Plateau came up with a series of laws to describe stable foam structures (foams that don't follow these laws tend to rearrange themselves until they do). They are:
1.Soap film surfaces are smooth.
2.The soap film curvature is continuous and constant along the entire a surface.
3.When three or more bubbles connect together, they will shift around until each line only contains three bubble-wall intersections – called a Plateau Border. With matching surface tensions all three angles are 120 degrees, the most efficient option.
4.Only four Plateau Borders can meet at a point.
Foams form in nature. Examples include: sea and river foams, and the foaming at the mouth of a rabid dog. There are fish, such as gourami and Siamese fighting fish, that blow a mass of bubbles coated in saliva to house their eggs. In the same spirit, some species of frogs make foam nests to lay their eggs in. These nests may be constructed in crevices, on the surface of water, or on forest floors.
Beyond bubble baths, soaps can be whipped to form a lather with bubbles so small they hardly can be seen. This idea was extended into modern shaving foams where a compressed gas is rapidly decompressed to expand a cream into a foam. Foams can also be hardened into permanent structures like insulation and flotation devices. Ceramic can be made into foams useful for acoustic insulation, absorption of environmental pollutants, and the filtration of molten metal alloys among other applications. Cement foams are used as a light-weight building material with good insulating capability. Even metals can be manipulated into becoming a foam. Metal foams are used in exhaust mufflers as they a great at dampening noise. They also make efficient materials for heaters and heat exchangers since they have so much surface area.
We eat a lot of foams: they can add a light texture to an angel food cake, or be tasty in the form of whipped cream and meringues. Breads are often a foam as the yeast produces tiny bubbles of gas which causes the dough to rise. One of the best foams is the head that forms when a beer is poured into a glass; this foam is made by which is made by carbon dioxide bubble rising to the surface. For the best head on your glass of beer, chose a wheat beer instead of a barley beer.
Note: there is an optimum foam combination of wheat beer consumed in a bubble bath – use with caution.
Foams form when billions of tiny bubbles are packed together within a solid or a liquid. Irregular sized bubbles are common in all foams except the most idealized ones. Like what happens for individual bubbles (check out my bubble post), it's surface tension that helps keep a foam stable. Liquid foams break down eventually – there are even chemicals on the market to make this process go faster. Gas can diffuse from small bubbles into large ones and eventually out of the foam, or gravity can drain liquid out the bottom making bubbles so weak they pop on top.
A Belgian physicist, Joseph Plateau (1801-1883), figured out the basis of what we know about soap films and foams. His diverse interests also included spending time with moving image illusions like the action one sees when using a "flip-book". Back to soap film, Plateau came up with a series of laws to describe stable foam structures (foams that don't follow these laws tend to rearrange themselves until they do). They are:
1.Soap film surfaces are smooth.
2.The soap film curvature is continuous and constant along the entire a surface.
3.When three or more bubbles connect together, they will shift around until each line only contains three bubble-wall intersections – called a Plateau Border. With matching surface tensions all three angles are 120 degrees, the most efficient option.
4.Only four Plateau Borders can meet at a point.
Foams form in nature. Examples include: sea and river foams, and the foaming at the mouth of a rabid dog. There are fish, such as gourami and Siamese fighting fish, that blow a mass of bubbles coated in saliva to house their eggs. In the same spirit, some species of frogs make foam nests to lay their eggs in. These nests may be constructed in crevices, on the surface of water, or on forest floors.
Beyond bubble baths, soaps can be whipped to form a lather with bubbles so small they hardly can be seen. This idea was extended into modern shaving foams where a compressed gas is rapidly decompressed to expand a cream into a foam. Foams can also be hardened into permanent structures like insulation and flotation devices. Ceramic can be made into foams useful for acoustic insulation, absorption of environmental pollutants, and the filtration of molten metal alloys among other applications. Cement foams are used as a light-weight building material with good insulating capability. Even metals can be manipulated into becoming a foam. Metal foams are used in exhaust mufflers as they a great at dampening noise. They also make efficient materials for heaters and heat exchangers since they have so much surface area.
We eat a lot of foams: they can add a light texture to an angel food cake, or be tasty in the form of whipped cream and meringues. Breads are often a foam as the yeast produces tiny bubbles of gas which causes the dough to rise. One of the best foams is the head that forms when a beer is poured into a glass; this foam is made by which is made by carbon dioxide bubble rising to the surface. For the best head on your glass of beer, chose a wheat beer instead of a barley beer.
Note: there is an optimum foam combination of wheat beer consumed in a bubble bath – use with caution.
Monday, October 4, 2010
Tiny bubbles
Anyone remember the show “That's Incredible!”? It was on in the early 80's. I watched it as a kid (and yes I'm dating myself as the show went off the air in 1984). One episode I remember vividly: The hosts hyped how this man could make a square bubble, which I suppose what hosts are supposed to do. Since all the bubbles I had ever seen were round, I was really curious how a square bubble could be made (and I wasn't yet jaded about TV). I was expecting the square bubble to be free-floating by itself, so I was kinda disappointed in how it was done. The “performance artist” blew a bunch of connected bubbles (I forget how many). Next he took a long inhale from a cigarette, then stuck a straw between the connected bubbles and filled the space with the smoke. The filled space was in the form of a cube, and I felt tricked.
A short lived creation, soap bubbles hold a sphere of air with a thin film of soapy water which is formed by surface tension. Spherical shapes are preferred (really large bubbles can end up forming elongated shapes from air currents) because a sphere is the smallest surface area possible to contain a specific volume of air. The soap film surface tension is strong and flexible enough that waves can travel along the surface and is so thin the surface appears iridescent.
Surprisingly, soapy water has less surface tension that water alone and is needed to keep the bubble stable. As a bubble is formed, the soap film stretches decreasing the concentration of soap which increases the surface tension. This mechanism is called the 'Marangoni Effect' and occurs when a surface tension gradient (that is regions of greater and lesser surface tension) causes liquid to move away from areas of low surface tension. The soap acts as a stabilizer by letting the thinnest parts of the film to have the strongest surface tension thus keeping the bubble together.
What happens when two bubbles stick together? Well, they will arrange themselves in such a way that minimizes the surface area. Bubbles of different sizes will end up with a bulging internal wall into the larger on as smaller bubbles have higher internal pressure. If they are the same size, the internal wall will be flat - a phenomenon exploited by the cube-making bubble performer.
So sneaky internal bubble-wall cubes aren't so impressive. How about antibubbles ….
A short lived creation, soap bubbles hold a sphere of air with a thin film of soapy water which is formed by surface tension. Spherical shapes are preferred (really large bubbles can end up forming elongated shapes from air currents) because a sphere is the smallest surface area possible to contain a specific volume of air. The soap film surface tension is strong and flexible enough that waves can travel along the surface and is so thin the surface appears iridescent.
Surprisingly, soapy water has less surface tension that water alone and is needed to keep the bubble stable. As a bubble is formed, the soap film stretches decreasing the concentration of soap which increases the surface tension. This mechanism is called the 'Marangoni Effect' and occurs when a surface tension gradient (that is regions of greater and lesser surface tension) causes liquid to move away from areas of low surface tension. The soap acts as a stabilizer by letting the thinnest parts of the film to have the strongest surface tension thus keeping the bubble together.
What happens when two bubbles stick together? Well, they will arrange themselves in such a way that minimizes the surface area. Bubbles of different sizes will end up with a bulging internal wall into the larger on as smaller bubbles have higher internal pressure. If they are the same size, the internal wall will be flat - a phenomenon exploited by the cube-making bubble performer.
So sneaky internal bubble-wall cubes aren't so impressive. How about antibubbles ….
Thursday, September 30, 2010
Talking to politicians
I was volunteered to present a simplified version of my research to a group of visiting politicians yesterday. I was given exactly 3.5 minutes to present starting at 3:13 pm, I guess politicians live in a precise world (as we all expected they showed up somewhat late). So here is what I said:
The focus on my research in on how the tides interact with Fraser Ridge (a sub-surface bump in the Strait of Georgia). One really interesting effect is the formation of lee waves on the flood tide. Ocean waters tend to be stratified, which means the water is organized so the denser water sits lower than the less dense water. As tides move this water over an obstacle like Fraser Ridge, water is forced to speed up because of the constricted space and it gains momentum. On the lee side of the obstacle the momentum can carry less dense water down deep. Eventually, friction causes the water to slow and the less dense water bounces back up in a formation called a lee wave. Effects like these lee waves can provide an explanation why some coastal sites form productive ecosystems.
What is interesting about Fraser Ridge is that a rare glass sponge reef has made its home there. Glass sponge reefs were once common in the world's oceans at the same time that dinosaurs roamed the earth. Like the dinosaurs, we thought glass sponge reefs were extinct. In the 1990s, living glass sponge reefs were discovered in a few locations off the coast of BC and it turns out they make great habitat for young rock fish. Glass sponges are filter feeders fixed to the bottom so they need enough flow to bring in their food, remove their waste and keep them from being buried in sediment. Periodic formation of lee waves can provide the flow they need.
At my study site, a lee wave forms on each flood tide. The sponges have chosen to live on the steepest slope which corresponds to the strongest currents, turbulence and sharpest lee wave.
The focus on my research in on how the tides interact with Fraser Ridge (a sub-surface bump in the Strait of Georgia). One really interesting effect is the formation of lee waves on the flood tide. Ocean waters tend to be stratified, which means the water is organized so the denser water sits lower than the less dense water. As tides move this water over an obstacle like Fraser Ridge, water is forced to speed up because of the constricted space and it gains momentum. On the lee side of the obstacle the momentum can carry less dense water down deep. Eventually, friction causes the water to slow and the less dense water bounces back up in a formation called a lee wave. Effects like these lee waves can provide an explanation why some coastal sites form productive ecosystems.
What is interesting about Fraser Ridge is that a rare glass sponge reef has made its home there. Glass sponge reefs were once common in the world's oceans at the same time that dinosaurs roamed the earth. Like the dinosaurs, we thought glass sponge reefs were extinct. In the 1990s, living glass sponge reefs were discovered in a few locations off the coast of BC and it turns out they make great habitat for young rock fish. Glass sponges are filter feeders fixed to the bottom so they need enough flow to bring in their food, remove their waste and keep them from being buried in sediment. Periodic formation of lee waves can provide the flow they need.
At my study site, a lee wave forms on each flood tide. The sponges have chosen to live on the steepest slope which corresponds to the strongest currents, turbulence and sharpest lee wave.
Tuesday, September 28, 2010
Working comfortably in remote places
I've been reading 'Packing for Mars' and enjoying it greatly. The author describes the ups and downs of working in space. I've never worked in space, but I certainly can relate to some of the difficulties of working in remote, confined and difficult places. Add on top of that sleep deprivation, situations can become really dangerous and/or hilarious (for some reason sleep deprivation can leave me giggly).
When I first started doing field work in the army I made the mistake of thinking that if I only had a short time to sleep, I should just lay down with my boots on and attempt to sleep anywhere – like on a pile of rocks (looked comfortable in the dark) or the main thoroughfare for an army of ants. It didn't take me long to realize taking the time to blow up an air mattress, pull out a sleeping bag, and take my boots off would make me so much more comfortable.
I've spent months working out of tents, which can be comfortable especially if I've brought a cot. I've even packed a pillow when heading out to these places. A large tented camp can be just like a small town – hot food and showers are often available. If the ground isn't frozen, mud always makes an appearance (I've never lived in a tented camp in the desert but I suspect the issue then would be sand). Everything can end up covered in mud and without running water it is hard to clean up. I've had mud fill all the treads on my boots to the point where I just slip slide around.
Working on small boats exposes one to the weather, which is okay with the proper clothing and a warm place to spend the night (or day if working on the boat at night). Open boats aren't my favorite; on a nasty day it is nice to get inside if only for a few moments.
By far, my favorite field work space are full sized ships – I like knowing there is a comfortable place to sleep nearby especially if my shift is long. Hot showers (even if they are short), clean clothes and hot food prepared by someone else are other pluses. There are always people around on a ship which I find to be a comfort if we are out in the middle of the ocean or up in the arctic. The downside to working on a ship is my tendency towards sea sickness which can be dealt with.
Where ever I go, being comfortable appears to be a choice. It's never that much harder to make oneself more comfortable – start with taking the boots off.
When I first started doing field work in the army I made the mistake of thinking that if I only had a short time to sleep, I should just lay down with my boots on and attempt to sleep anywhere – like on a pile of rocks (looked comfortable in the dark) or the main thoroughfare for an army of ants. It didn't take me long to realize taking the time to blow up an air mattress, pull out a sleeping bag, and take my boots off would make me so much more comfortable.
I've spent months working out of tents, which can be comfortable especially if I've brought a cot. I've even packed a pillow when heading out to these places. A large tented camp can be just like a small town – hot food and showers are often available. If the ground isn't frozen, mud always makes an appearance (I've never lived in a tented camp in the desert but I suspect the issue then would be sand). Everything can end up covered in mud and without running water it is hard to clean up. I've had mud fill all the treads on my boots to the point where I just slip slide around.
Working on small boats exposes one to the weather, which is okay with the proper clothing and a warm place to spend the night (or day if working on the boat at night). Open boats aren't my favorite; on a nasty day it is nice to get inside if only for a few moments.
By far, my favorite field work space are full sized ships – I like knowing there is a comfortable place to sleep nearby especially if my shift is long. Hot showers (even if they are short), clean clothes and hot food prepared by someone else are other pluses. There are always people around on a ship which I find to be a comfort if we are out in the middle of the ocean or up in the arctic. The downside to working on a ship is my tendency towards sea sickness which can be dealt with.
Where ever I go, being comfortable appears to be a choice. It's never that much harder to make oneself more comfortable – start with taking the boots off.
Thursday, September 16, 2010
Water drops on kale

I was out in my garden this morning in the few moments of sun before more rain and I noticed beads of rain water had formed on the kale leaves. See how the veins on the leaf are moved when looked at through the water? See the sky reflection? If I had leaned close enough, my reflection would probably be visible too.
Wednesday, September 15, 2010
Sticky Honey
At first honey flows as a thick glop that evolves into an seemingly infinite stream. I never bother to wait long enough for it all to come off my measuring spoon, instead I just lick it – actually I could just lick spoons of honey without putting it into anything (like everyone else, I'm hardwired to like sweet things). Honey is sweet in a complex way I find intriguing. I'm always on the lookout for different types of honey to try. Recently, I bought a big plastic tub of clover honey on my trip east. Years ago, while wandering around downtown Munich, I found an entire shop devoted to different types of honey – I was amazed by the sheer number of types of honey: lavender, clover, buckwheat, avocado, heather and the list could go on. I think people really like honey.
Honey has been consumed since ancient times, likely as far back as 10,000 years ago or more. At one time honey was often the only sweetener available. When the tomb of King Tutankhamun was found in 1922, a pot of honey was discovered inside that was still edible (I'm not sure I'd go for potentially cursed mummy honey). On top of sweetening, honey has been used medicinally for eons. Modern studies have shown that honey helps healing wounds, it even provides a soothing effect when applied to burns. It also is a possible treatment for gingivitis, cataracts, ulcers and more.
According to 'The Flavor Bible', honey is a moderately loud flavor considered 'rustic' that goes with both the savory and the sweet. Every honey made tastes unique because the flavour of honey is determined by the flower the nectar came from, and there are almost an infinite number of possible flower combinations. Bees can be picky about what flowers they use. In one study, beehives were situated in the middle of avocado orchards while the avocado trees were blooming. Avocados produce a lot of nectar, so it should have been a win-win for the trees and bees. It turned out the bees preferred nectar from flowers surrounding the orchard.
Once nectar is brought back to the hive, the bees ingest and regurgitate the nectar multiple times until it is partially digested. At this point, it's stored in the honey comb while worker bees fan it with their wings until enough water evaporates. When the water content is low enough, honey will never spoil. When finished, honey has the following composition: 17.1% water, 82.4% total carbohydrate and 0.5% proteins, amino acids, vitamins and minerals.
At 64 calories per tablespoon, honey is a good source of nutrition. Honey colour is an indication of how strong the honey will taste. Light honeys are mild and dark honeys are stronger. It turns out that the darker honeys often have more antioxidants and potassium, but beware, honey can darken during shipping and storage.
What I think is cool about honey (beyond snacking possibilities) is that it's a non-Newtonian fluid. A non-Newtonian fluid is a fancy way to say that it doesn't respond evenly when poured in contrast to a Newtonian fluid like water. If you take a spoon of water and turn it over the water will flow out at an even rate. If you take a spoon of honey and turn it over, at first it will just bulge then a thick stream will slowly descend downwards. Over time the stream will speed up and thin.
In a non-Newtonian fluid the relationship between shear stress (pushing parallel to flow) and strain rate (how fast it deforms) is non-linear. That is, a simple number, usually viscosity (resistance or thickness of the fluid), can't be used to relate shear stress and strain rate together. To add complexity, this effect can even vary with time. The large molecules of the honey form links with each other that have an elasticity to them and can actually counteract for a while forces like gravity. Eventually, the flow overcomes this resistance and links will break. Other links will form and this non-linear effect will persist.
I'm going to keep looking for different types of honey when I travel and I won't expect it to come out of the jar easily.
Honey has been consumed since ancient times, likely as far back as 10,000 years ago or more. At one time honey was often the only sweetener available. When the tomb of King Tutankhamun was found in 1922, a pot of honey was discovered inside that was still edible (I'm not sure I'd go for potentially cursed mummy honey). On top of sweetening, honey has been used medicinally for eons. Modern studies have shown that honey helps healing wounds, it even provides a soothing effect when applied to burns. It also is a possible treatment for gingivitis, cataracts, ulcers and more.
According to 'The Flavor Bible', honey is a moderately loud flavor considered 'rustic' that goes with both the savory and the sweet. Every honey made tastes unique because the flavour of honey is determined by the flower the nectar came from, and there are almost an infinite number of possible flower combinations. Bees can be picky about what flowers they use. In one study, beehives were situated in the middle of avocado orchards while the avocado trees were blooming. Avocados produce a lot of nectar, so it should have been a win-win for the trees and bees. It turned out the bees preferred nectar from flowers surrounding the orchard.
Once nectar is brought back to the hive, the bees ingest and regurgitate the nectar multiple times until it is partially digested. At this point, it's stored in the honey comb while worker bees fan it with their wings until enough water evaporates. When the water content is low enough, honey will never spoil. When finished, honey has the following composition: 17.1% water, 82.4% total carbohydrate and 0.5% proteins, amino acids, vitamins and minerals.
At 64 calories per tablespoon, honey is a good source of nutrition. Honey colour is an indication of how strong the honey will taste. Light honeys are mild and dark honeys are stronger. It turns out that the darker honeys often have more antioxidants and potassium, but beware, honey can darken during shipping and storage.
What I think is cool about honey (beyond snacking possibilities) is that it's a non-Newtonian fluid. A non-Newtonian fluid is a fancy way to say that it doesn't respond evenly when poured in contrast to a Newtonian fluid like water. If you take a spoon of water and turn it over the water will flow out at an even rate. If you take a spoon of honey and turn it over, at first it will just bulge then a thick stream will slowly descend downwards. Over time the stream will speed up and thin.
In a non-Newtonian fluid the relationship between shear stress (pushing parallel to flow) and strain rate (how fast it deforms) is non-linear. That is, a simple number, usually viscosity (resistance or thickness of the fluid), can't be used to relate shear stress and strain rate together. To add complexity, this effect can even vary with time. The large molecules of the honey form links with each other that have an elasticity to them and can actually counteract for a while forces like gravity. Eventually, the flow overcomes this resistance and links will break. Other links will form and this non-linear effect will persist.
I'm going to keep looking for different types of honey when I travel and I won't expect it to come out of the jar easily.
Friday, September 10, 2010
Making Blue - part 2
Further to my last post, another synthesized blue lurks in my paint box – Prussian Blue. It is a complex dark blue pigment that was first synthesized around 1706 by the paint maker Diesbach (whose first name I couldn't find) in Berlin. Since its discovery, Prussian Blue has been used extensively in making paint, and is the traditional "blue" in blueprints. Strangely, It has been used as an antidote for certain kinds of heavy metal poisoning – perhaps a story for another day.
The synthetic Prussian Blue filled a gap left by the loss of knowledge of how to make Egyptian Blue. It is a stable and relatively light-fast blue that is cheaper than ultramarine made from lapis lazuli. Artists were waiting for a pigment like this, so within two years of it's discovery it was already being traded across Europe. Prussian Blue is a strong colour that tends towards black or dark purple when mixed into oil paints. Interestingly, the particle size of the pigment creates the exact hue.
Prussian Blue is a complex chemical including iron and cyanide. It's not particularly toxic because the cyanide is bound tightly to the iron. I was surprised to learn about the number of applications where this pigment is used. In medicine, Prussian Blue is used to detect iron in biopsies like bone marrow. It is also the basis for laundry bluing, that is, it's used to add a slight hint of blue to someone's washing to combat yellowing of whites.
Once children's crayons contained a Prussian Blue, but now that has been changed to Midnight Blue. It has been a long time since I've looked at crayon colours – I think the last time was when I melted them to colour wax for candle making.
The synthetic Prussian Blue filled a gap left by the loss of knowledge of how to make Egyptian Blue. It is a stable and relatively light-fast blue that is cheaper than ultramarine made from lapis lazuli. Artists were waiting for a pigment like this, so within two years of it's discovery it was already being traded across Europe. Prussian Blue is a strong colour that tends towards black or dark purple when mixed into oil paints. Interestingly, the particle size of the pigment creates the exact hue.
Prussian Blue is a complex chemical including iron and cyanide. It's not particularly toxic because the cyanide is bound tightly to the iron. I was surprised to learn about the number of applications where this pigment is used. In medicine, Prussian Blue is used to detect iron in biopsies like bone marrow. It is also the basis for laundry bluing, that is, it's used to add a slight hint of blue to someone's washing to combat yellowing of whites.
Once children's crayons contained a Prussian Blue, but now that has been changed to Midnight Blue. It has been a long time since I've looked at crayon colours – I think the last time was when I melted them to colour wax for candle making.
Tuesday, September 7, 2010
Making Blue – or what to do if you don't have enough lapis lazuli
'Through the atmosphere the universe tones towards us in the colour blue and according to the thickness of the air, takes on every grade of blue until it goes over to black-violet on the mountain tops'
-Goethe 'Theory of Colours'
The above quote makes me think of the Van Gogh painting 'The Starry Night' – one of my favorites. I love the shades of blue (I love the swirls too but, that makes up a different story). So what makes paint blue? Typical pigments used include Azurite, Cerulean Blue, Cobalt Blue, Prussian Blue and Ultramarine which was once made from lapis lazuli.
Although there are other natural blues that can be used in pigments, lapis lazuli intrigues me the most. Years ago, I read a book on natural colours where the author journeyed to Afghanistan (in safer times) to find lapis lazuli. I don't have the book at hand, so I can't quote from it, but ever since then I've thought lapis lazuli had a fantastic story, I even like saying 'lapis lazuli'. Powdered lapis was used as eyeshadow by Cleopatra – what could be more exotic than that?
Lapis lazuli has always been prized for its intense blue color. It has been mined from Afghanistan for over 6,000 years and there are other sources around Lake Baikal in Siberia. Lapis lazuli is classified as a rock composed of more than one mineral. Since it polishes well, it can be made into jewelry, carvings, boxes, mosaics, ornaments, and vases. In ancient Egypt, lapis lazuli was favored for inclusion on amulets and ornaments such as scarabs. To answer my paint question, lapis lazuli was also ground and processed to make the pigment ultramarine.
So what if lapis lazuli wasn't available (or too expensive)? Before modern synthetic colours became available there were several options.
Egyptian Blue is a pigment that was made and used by Egyptians for thousands of years and may even be the first synthetic pigment. In Egyptian it's called 'hsbd-iryt', which translates to 'artificial lapis lazuli'. Although it's only one of many components, copper is what makes Egyptian Blue blue. The exact hue of blue can range from light to dark depending on how it is made. Egyptian Blue coloured stone, wood, plaster, papyrus, and canvas. It was also used in objects like cylinder seals, beads, scarabs, inlays, pots and statuettes. Unfortunately, when the Roman era ended, knowledge on how to make Egyptian Blue was lost. Egyptian blue has been found on objects from all over the Roman Empire and may have been independently discovered in places like ancient China.
At least 2,000 years ago a synthetic blue turned up in China. Chinese Blue and Egyptian Blue have the same basic structure and have very similar properties. The difference is that Egyptian Blue contains calcium where Chinese Blue has barium. Was this Chinese Blue produced from knowledge of Egyptian Blue making it's way along the silk road? There are theories that lean both ways.
Another ancient blue comes from pre-columbian mesoamerica and examples are still blue today. Maya Blue is a organic-inorganic hybrid that was made by heating indigo and a fibrous clay together. This method worked so well it is an active area of research today.
Back to lapis lazuli. Lapis lazuli's use as a pigment in oil paint ended in the early 19th century when a chemically identical synthetic variety, often called French Ultramarine, became available.
-Goethe 'Theory of Colours'
The above quote makes me think of the Van Gogh painting 'The Starry Night' – one of my favorites. I love the shades of blue (I love the swirls too but, that makes up a different story). So what makes paint blue? Typical pigments used include Azurite, Cerulean Blue, Cobalt Blue, Prussian Blue and Ultramarine which was once made from lapis lazuli.
Although there are other natural blues that can be used in pigments, lapis lazuli intrigues me the most. Years ago, I read a book on natural colours where the author journeyed to Afghanistan (in safer times) to find lapis lazuli. I don't have the book at hand, so I can't quote from it, but ever since then I've thought lapis lazuli had a fantastic story, I even like saying 'lapis lazuli'. Powdered lapis was used as eyeshadow by Cleopatra – what could be more exotic than that?
Lapis lazuli has always been prized for its intense blue color. It has been mined from Afghanistan for over 6,000 years and there are other sources around Lake Baikal in Siberia. Lapis lazuli is classified as a rock composed of more than one mineral. Since it polishes well, it can be made into jewelry, carvings, boxes, mosaics, ornaments, and vases. In ancient Egypt, lapis lazuli was favored for inclusion on amulets and ornaments such as scarabs. To answer my paint question, lapis lazuli was also ground and processed to make the pigment ultramarine.
So what if lapis lazuli wasn't available (or too expensive)? Before modern synthetic colours became available there were several options.
Egyptian Blue is a pigment that was made and used by Egyptians for thousands of years and may even be the first synthetic pigment. In Egyptian it's called 'hsbd-iryt', which translates to 'artificial lapis lazuli'. Although it's only one of many components, copper is what makes Egyptian Blue blue. The exact hue of blue can range from light to dark depending on how it is made. Egyptian Blue coloured stone, wood, plaster, papyrus, and canvas. It was also used in objects like cylinder seals, beads, scarabs, inlays, pots and statuettes. Unfortunately, when the Roman era ended, knowledge on how to make Egyptian Blue was lost. Egyptian blue has been found on objects from all over the Roman Empire and may have been independently discovered in places like ancient China.
At least 2,000 years ago a synthetic blue turned up in China. Chinese Blue and Egyptian Blue have the same basic structure and have very similar properties. The difference is that Egyptian Blue contains calcium where Chinese Blue has barium. Was this Chinese Blue produced from knowledge of Egyptian Blue making it's way along the silk road? There are theories that lean both ways.
Another ancient blue comes from pre-columbian mesoamerica and examples are still blue today. Maya Blue is a organic-inorganic hybrid that was made by heating indigo and a fibrous clay together. This method worked so well it is an active area of research today.
Back to lapis lazuli. Lapis lazuli's use as a pigment in oil paint ended in the early 19th century when a chemically identical synthetic variety, often called French Ultramarine, became available.
Sunday, September 5, 2010
Origami Shrimp

In my aquarium I have a number of Amano Shrimp who keep the place clean. Amano Shrimp originate from South Eastern Asia and have clear bodies about a knuckle long with wine-red spots. They have an interesting life cycle in that they are a fresh water shrimp whose larvae require salt water to live. In the wild they must migrate up and down rivers throughout their lives. When I give my fish flake food, these shrimp always dart forward and snatch the largest flakes. They then fold up the flakes into what looks like origami shapes before munching on them. I assume they fold their food this way to make a large flake less cumbersome to move with – or perhaps they just like origami.
Origami is a Japanese art of folding paper. According to wikipedia: The goal of this art is to transform a flat sheet of material into a finished sculpture through folding and sculpting techniques, and as such the use of cuts or glue are not considered to be origami. I have a number of origami how-to books from which I could make creatures from sea stars to giraffes – I don't do a lot of folding, I just have some books.
Origami is an applied geometry that has practical applications beyond making pretty cranes. Origami folds can be planned mathematically as there are a limited number of ways a piece of paper can be folded. Computational origami extends the math to optimize folds for practical like folding an airbag for car or finding an efficient way to fold solar panels to make the journey to space.
Interesting links here and here.
Saturday, September 4, 2010
More on candles
'There is no better, there is no more open door which you can enter into the study of natural philosophy than by considering the physical phenomenon of a candle'
- Michael Faraday (1791-1867)
Faraday was a physicist and chemist, best known for his contributions in the areas of electricity and magnetism. Two units of measures are named for him: the Faraday (unit of electrical charge) and the Farad (unit of electrical capacitance). Faraday also spent time on projects such as lighthouse construction and operation, and protecting metal ship hulls from corrosion. He gave tips on the cleaning and protection of artwork. On top of these and other activities, he delivered a series of public lectures and wrote for the general public. Around 1860, Faraday gave a successful series of lectures on the chemistry and physics of flames called 'The Chemical History of a Candle', a transcript of which I stumbled upon recently.
On describing a method of making candles, he explained: 'The fat or tallow is first boiled with quick-lime and made into soap, and then the soap is decomposed by sulphuric acid, which takes away the lime, and leaves the fat rearranged as stearic acid, while a quantity of Glycern is produced at the same time ... The oil is then pressed out ... and at last you have left that substance which is melted and cast into candles'
Makes me tired just reading that! Candles can also be made by the dip method I've described before or from bees wax. In Faraday's day sperm candles were made from purified oil found within the head cavities of sperm whales and paraffin candles were made from paraffin somehow obtained from bogs in Ireland (how exactly this was done was not mentioned – I'm curious so I may look this up later).
A burning candle is a chemical reaction that turns wax and oxygen into carbon dioxide and water while letting off heat and light. Soot isn't a product of this chemical reaction, instead it is incompletely burned carbon. Once lit, how does a candle get fuel to sustain itself?
The heat of the flame melts a pool of wax. This wax is then drawn to the flame by capillary action – the wick just provides a way to get wax to flame. Capillary action is a process where liquid can rise, seemingly against the force of gravity, and it is common in the world around us. For example, the transport of fluids in plants uses capillary action. If you were to put a freshly cut celery stick into a cup of water that had purple food colouring in it, you would end up with purple celery. Capillary action occurs in thin tubes or within the weave of a candlewick as a result of inter-molecular attractive forces between the liquid and solid surrounding surfaces.
Molecules within a liquid are attracted to one another, this is called cohesion, which manifests as surface tension. Because of cohesion, the most efficient shape for a liquid is a sphere, which is why raindrops are round. When a liquid touches a solid material (like the wick) that attraction now occurs with the solid material – this is called adhesion. If adhesion is greater than cohesion the surface will curve up at the boundary like the meniscus formed when water is in a glass. Alternatively, if the adhesion is less than cohesion, then the surface will curve down. So, if the cohesion of water molecules and adhesion to a solid surface act together (which would happen in a thin space) the liquid would be drawn up – the thinner the space, the higher the liquid would rise. And that's how wax gets to the flame.
By the way, Faraday's idea of using a common thing like a candle flame to teach science is still used.
- Michael Faraday (1791-1867)
Faraday was a physicist and chemist, best known for his contributions in the areas of electricity and magnetism. Two units of measures are named for him: the Faraday (unit of electrical charge) and the Farad (unit of electrical capacitance). Faraday also spent time on projects such as lighthouse construction and operation, and protecting metal ship hulls from corrosion. He gave tips on the cleaning and protection of artwork. On top of these and other activities, he delivered a series of public lectures and wrote for the general public. Around 1860, Faraday gave a successful series of lectures on the chemistry and physics of flames called 'The Chemical History of a Candle', a transcript of which I stumbled upon recently.
On describing a method of making candles, he explained: 'The fat or tallow is first boiled with quick-lime and made into soap, and then the soap is decomposed by sulphuric acid, which takes away the lime, and leaves the fat rearranged as stearic acid, while a quantity of Glycern is produced at the same time ... The oil is then pressed out ... and at last you have left that substance which is melted and cast into candles'
Makes me tired just reading that! Candles can also be made by the dip method I've described before or from bees wax. In Faraday's day sperm candles were made from purified oil found within the head cavities of sperm whales and paraffin candles were made from paraffin somehow obtained from bogs in Ireland (how exactly this was done was not mentioned – I'm curious so I may look this up later).
A burning candle is a chemical reaction that turns wax and oxygen into carbon dioxide and water while letting off heat and light. Soot isn't a product of this chemical reaction, instead it is incompletely burned carbon. Once lit, how does a candle get fuel to sustain itself?
The heat of the flame melts a pool of wax. This wax is then drawn to the flame by capillary action – the wick just provides a way to get wax to flame. Capillary action is a process where liquid can rise, seemingly against the force of gravity, and it is common in the world around us. For example, the transport of fluids in plants uses capillary action. If you were to put a freshly cut celery stick into a cup of water that had purple food colouring in it, you would end up with purple celery. Capillary action occurs in thin tubes or within the weave of a candlewick as a result of inter-molecular attractive forces between the liquid and solid surrounding surfaces.
Molecules within a liquid are attracted to one another, this is called cohesion, which manifests as surface tension. Because of cohesion, the most efficient shape for a liquid is a sphere, which is why raindrops are round. When a liquid touches a solid material (like the wick) that attraction now occurs with the solid material – this is called adhesion. If adhesion is greater than cohesion the surface will curve up at the boundary like the meniscus formed when water is in a glass. Alternatively, if the adhesion is less than cohesion, then the surface will curve down. So, if the cohesion of water molecules and adhesion to a solid surface act together (which would happen in a thin space) the liquid would be drawn up – the thinner the space, the higher the liquid would rise. And that's how wax gets to the flame.
By the way, Faraday's idea of using a common thing like a candle flame to teach science is still used.
Tuesday, August 31, 2010
The case of the organic salt
I live in a city with a lot of organic food shops which gives me a fabulous amount of food choices that I didn't have when I lived in other places. But, I have to admit some things for sale at these shops don't make a lot of sense – like organic apples from New Zealand (a hemisphere away from my home) for sale at the same time I can get them fresh and ripe from a local orchard.
Because we were curious if we would actually find it, my significant other and I decided to look for organic salt. Our theory was that organic salt would be found if we looked for it. Remember, that by definition salt is inorganic because it was never living and is not made up of any carbon – it's just sodium and chloride.
We checked two shops and didn't find our salt. But, at the third shop we found it – a package labeled 'organic salt'. The only ingredient was salt. I didn't buy any as I can make due with the plain old inorganic stuff (and I'm a little confused about what would make salt organic). When I got home I did google search for 'organic salt'. Pages of products come up ranging from foods to bath products. I can't stop myself from thinking manufactures, including the organic shop since the organic salt we found there was a shop brand, are just jumping on the organic band wagon and using it as a buzz word.
Because we were curious if we would actually find it, my significant other and I decided to look for organic salt. Our theory was that organic salt would be found if we looked for it. Remember, that by definition salt is inorganic because it was never living and is not made up of any carbon – it's just sodium and chloride.
We checked two shops and didn't find our salt. But, at the third shop we found it – a package labeled 'organic salt'. The only ingredient was salt. I didn't buy any as I can make due with the plain old inorganic stuff (and I'm a little confused about what would make salt organic). When I got home I did google search for 'organic salt'. Pages of products come up ranging from foods to bath products. I can't stop myself from thinking manufactures, including the organic shop since the organic salt we found there was a shop brand, are just jumping on the organic band wagon and using it as a buzz word.
Monday, August 30, 2010
Flow over bumps
As Fluid flows over a bump surface disturbances, such as waves, develop potentially extending both up and downstream. The form these disturbances take depends on fluid velocity and fluid depth which can be combined together in the Froude (F) number. The Froude number equals the fluid velocity over the square root of gravity times fluid depth. Because it has no dimensions, the Froude number allows flow in dramatically different circumstances to be compared, for example, atmospheric flow over a mountain range could be compared to tap water flow from your kitchen sink.
Naval architecture provided the original application for the Froude number. Here the hull length of a ship is used instead of fluid depth. It's important because, the Froude number relates to the ship's drag or resistance to moving through the water. This number is named after William Froude (1810-1879) who experimented on ship's hulls in his large fluid tank. William developed towing tank techniques in his efforts to model frictional drag on ships. However, he didn't discover the dynamical relationship between the fluid velocity and hull length. It was Ferdinand Reech (1805-1880) who first described this relationship and used it for testing ships and propellers around 1852. It's likely that this relationship's roots reside with even earlier French mathematicians.
So what does the Froude number tell us? When F is smaller than one, flow over the bump is 'subcritical'. Waves on the surface can travel upstream, meaning that downstream conditions affect the flow upstream. For example, when a pebble is tossed into the water of a flowing stream, the resulting ripples propagate both upstream and downstream. When F is larger than 1, flow is 'supercritical'. In this case, no surface disturbance can travel upstream. The ripples created by a pebble tossed in downstream cannot overcome the speed of the water. The flow upstream is not changed. When F is equal to one the flow is 'critical'. This is the point of transition from subcritical to supercritical effects.
Now, back to flow over a bump. As subcritical water is pushed over the bump, squeezing takes place because the water is now shallower and the same amount of water is flowing through. This forces the water to speed up over the bump and transition to supercritical. This faster water crosses over to the other side of the bump, where it's again deeper and slower moving. When the fast flowing water reaches the slower water it abruptly slows and waves form. Since the water is moving too quickly to allow waves to propagate upstream, (because it is supercritical) these waves build up, forming a sudden water level increase that can be standing still in the flowing water. This is called a hydraulic jump, a non-linear effect and can be observed in a kitchen sink or in water passing over a weir. Mathematically, a hydraulic jump is a discontinuity, however in the real world viscosity makes it a region of rapid change instead.
The greater the Froude number is, the more pronounced the jump will be. For initial flow speeds slightly above the critical speed, the transition appears as an undulating wave. As flow speed increases, the Froude number also increases and the transition becomes stronger eventually developing a more abrupt shape. When the speed is high enough, the transition front will break and curl back upon itself. At this point, the jump may contain violent turbulence, eddying, air entrainment, and surface waves. Turbulence removes the extra energy, allowing the flow to transition from supercritical back to subcritical.
Naval architecture provided the original application for the Froude number. Here the hull length of a ship is used instead of fluid depth. It's important because, the Froude number relates to the ship's drag or resistance to moving through the water. This number is named after William Froude (1810-1879) who experimented on ship's hulls in his large fluid tank. William developed towing tank techniques in his efforts to model frictional drag on ships. However, he didn't discover the dynamical relationship between the fluid velocity and hull length. It was Ferdinand Reech (1805-1880) who first described this relationship and used it for testing ships and propellers around 1852. It's likely that this relationship's roots reside with even earlier French mathematicians.
So what does the Froude number tell us? When F is smaller than one, flow over the bump is 'subcritical'. Waves on the surface can travel upstream, meaning that downstream conditions affect the flow upstream. For example, when a pebble is tossed into the water of a flowing stream, the resulting ripples propagate both upstream and downstream. When F is larger than 1, flow is 'supercritical'. In this case, no surface disturbance can travel upstream. The ripples created by a pebble tossed in downstream cannot overcome the speed of the water. The flow upstream is not changed. When F is equal to one the flow is 'critical'. This is the point of transition from subcritical to supercritical effects.
Now, back to flow over a bump. As subcritical water is pushed over the bump, squeezing takes place because the water is now shallower and the same amount of water is flowing through. This forces the water to speed up over the bump and transition to supercritical. This faster water crosses over to the other side of the bump, where it's again deeper and slower moving. When the fast flowing water reaches the slower water it abruptly slows and waves form. Since the water is moving too quickly to allow waves to propagate upstream, (because it is supercritical) these waves build up, forming a sudden water level increase that can be standing still in the flowing water. This is called a hydraulic jump, a non-linear effect and can be observed in a kitchen sink or in water passing over a weir. Mathematically, a hydraulic jump is a discontinuity, however in the real world viscosity makes it a region of rapid change instead.
The greater the Froude number is, the more pronounced the jump will be. For initial flow speeds slightly above the critical speed, the transition appears as an undulating wave. As flow speed increases, the Froude number also increases and the transition becomes stronger eventually developing a more abrupt shape. When the speed is high enough, the transition front will break and curl back upon itself. At this point, the jump may contain violent turbulence, eddying, air entrainment, and surface waves. Turbulence removes the extra energy, allowing the flow to transition from supercritical back to subcritical.
Tuesday, August 24, 2010
Playing With Food
I was searching the internet for information on non-Newtonian fluids (which I'll write about in detail later) and came across a wealth of articles regarding the study of food textures. There is even a journal devoted to these texture studies. A quick look at the authors showed most worked for major food companies and the focus was on processed food (which I avoid or process myself) – nevertheless, I found myself drawn into their studies.
Some studies take the same approach as oceanography (my field) by considering a unit volume and the stresses that act on it. I'm delighted to know this has been applied to different gummy formulas, along with studying their attractive transparent appearance and textural attributes – for if I could eat one candy item until the end of time it would be gummy candies.
I also found a study out of India that looked at replacing a percentage of wheat flour in bread with a mixture of ground-up soya beans, oats, fenugreek seeds, flax seeds, and sesame seeds. The resulting loaves of bread were examined under a scanning electron microscope along with being tasted. Replacing 15% of the flour with this mixture resulted in bread that was just right. In another Indian study, the addition of ground flax seed to muffins was tested by a panel of six. This panel judged the muffins on crust colour, crumb colour, grain, texture, taste and over-all quality. Food has become complex!
I assume these studies happen because the processed food industry is big business, and I'm pleased to read that nutrition is included. One article from the Journal of Food Engineering declared 'not all foods are processed beginning with pure components; in fact most food products have their origin in the animal and vegetable kingdom.' I have to admit that the idea of foods (other than salt) that don't come from the animal and vegetable kingdom (assuming they are including fungi – mushrooms are tasty) worry me a lot. I wonder if bread analyzed under a scanning electron microscope really is any better that the bread I bake in my kitchen.
Some studies take the same approach as oceanography (my field) by considering a unit volume and the stresses that act on it. I'm delighted to know this has been applied to different gummy formulas, along with studying their attractive transparent appearance and textural attributes – for if I could eat one candy item until the end of time it would be gummy candies.
I also found a study out of India that looked at replacing a percentage of wheat flour in bread with a mixture of ground-up soya beans, oats, fenugreek seeds, flax seeds, and sesame seeds. The resulting loaves of bread were examined under a scanning electron microscope along with being tasted. Replacing 15% of the flour with this mixture resulted in bread that was just right. In another Indian study, the addition of ground flax seed to muffins was tested by a panel of six. This panel judged the muffins on crust colour, crumb colour, grain, texture, taste and over-all quality. Food has become complex!
I assume these studies happen because the processed food industry is big business, and I'm pleased to read that nutrition is included. One article from the Journal of Food Engineering declared 'not all foods are processed beginning with pure components; in fact most food products have their origin in the animal and vegetable kingdom.' I have to admit that the idea of foods (other than salt) that don't come from the animal and vegetable kingdom (assuming they are including fungi – mushrooms are tasty) worry me a lot. I wonder if bread analyzed under a scanning electron microscope really is any better that the bread I bake in my kitchen.
Subscribe to:
Posts (Atom)